Coronavirus: rush to publish may irreversibly harm patients
Medical ethics researchers have identified dozens of scientific papers as being discredited during the rush to publish papers amid the COVID-19 pandemic.
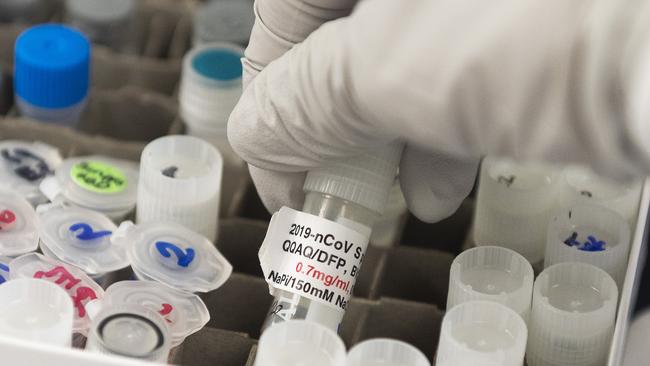
Medical ethics researchers have identified dozens of scientific papers as being discredited amid data falsification and misinterpretation, invalid conclusions and flawed methodology during the rush to publish papers during the COVID-19 pandemic.
As the novel coronavirus swept the world, there was a rush to publish scientific papers on preprint servers, upending the previous practice of waiting for peer review before findings were published.
While there have been significant upsides to the new era of open science, Bond University adjunct professor Katrina Bramstedt has warned that adverse medical results could result if clinicians rely on the conclusions of erroneous scientific papers in treating COVID-19 patients.
“Preprint platforms do routinely advise their readers not to use their content for clinical decision-making, but the latter cannot be ruled out, especially in the situation of a pandemic with high rates of morbidity and mortality,” Professor Bramstedt said.
“Patient harm that is significant, permanent and irreversible could result from using faulty research results.”
Professor Bramstedt wrote a paper entitled The Carnage of substandard research during the COVID-19 pandemic: a call for quality, published on Friday in the Journal of Medical Ethics.
The study identified 33 scientific papers that had been retracted, withdrawn or “noted with concern”. Some of the discredited papers were published in the world’s top scientific journals, including The Lancet and The New England Journal of Medicine.
In May, The Lancet was forced to retract a high-profile paper that purported to show the administration of hydroxychloroquine was associated with higher death rates in patients and significant levels of cardiac toxicity. The study was revealed to be based on dodgy data supplied by a shadowy healthcare analytics company, Surgisphere.
Professor Bramstedt found more than half the discredited papers published during the pandemic originated in Asia, mostly China. French journals had also published papers of concern.
“Research normally occurs at the speed of a marathon but during a pandemic the pace is more like a sprint,” she said in her study. “No research team is exempt from the pressures and speed at which COVID-19 research is occurring. This can increase the risk of honest error as well as misconduct.”
Almost 4000 papers related to COVID-19 had been uploaded to preprint servers in recent months, before being peer-reviewed. That represents about a quarter of all scientific articles on the virus.
However, Queensland University of Technology professor Virginia Barbour, director of the Australasian Open Access Strategy Group, said open access outside of subscriptions to scientific papers during the COVID-19 pandemic had transformed science.
“The advantage of them being online and immediate, even if they’re preliminary, is that they can get very rapid scrutiny,” she said. “High-profile cases of retractions (such as seen in COVID-19) are disturbing, but they also show that a key part of the scientific process, systematic review and evidence synthesis, is working.”
Australia’s COVID-19 Clinical Evidence Taskforce critically appraises scientific literature and develops evidence-based clinical guidelines for clinicians on treating coronavirus. Taskforce executive director Julian Elliott said all scientific papers were systematically assessed before being reviewed by an expert panel.
“If a study is available as preprint this becomes even more important, because any inconsistencies that peer review might have otherwise identified now fall on us to pick up,” he said.
While agreeing that retractions “are always a problem, as the scientific process is based on trust”, he said high-profile retractions, while disturbing, showed systematic review and evidence synthesis were working.







To join the conversation, please log in. Don't have an account? Register
Join the conversation, you are commenting as Logout