Health funds query a cure for back pain subject to lawsuits
Tens of thousands of Australians could be at risk of dangerous side effects from the misuse of a product used in spinal surgery, which has been involved in 10,000 US lawsuits.
Health
Don't miss out on the headlines from Health. Followed categories will be added to My News.
Exclusive: A medical bone graft - which has resulted in more than 10,000 legal settlements in the US and in some cases contributed to serious injury - continues to be used in Australia.
Up to 40,000 Australians could be at risk of adverse events from the off label use of a Medtronic bone graft product, which is approved by Australia’s medical regulator the Therapeutic Goods Administration.
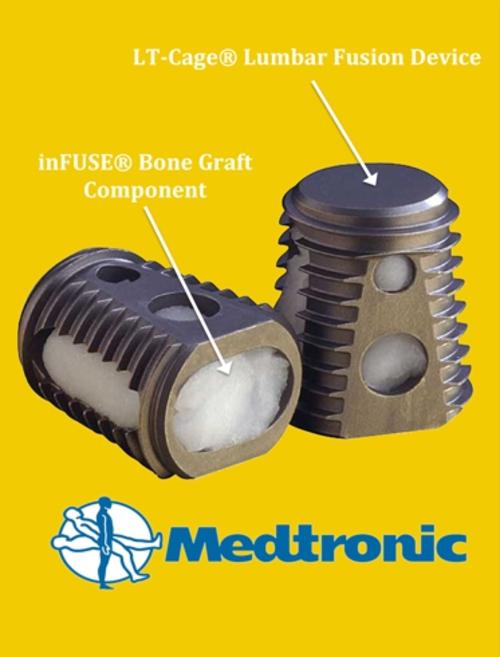
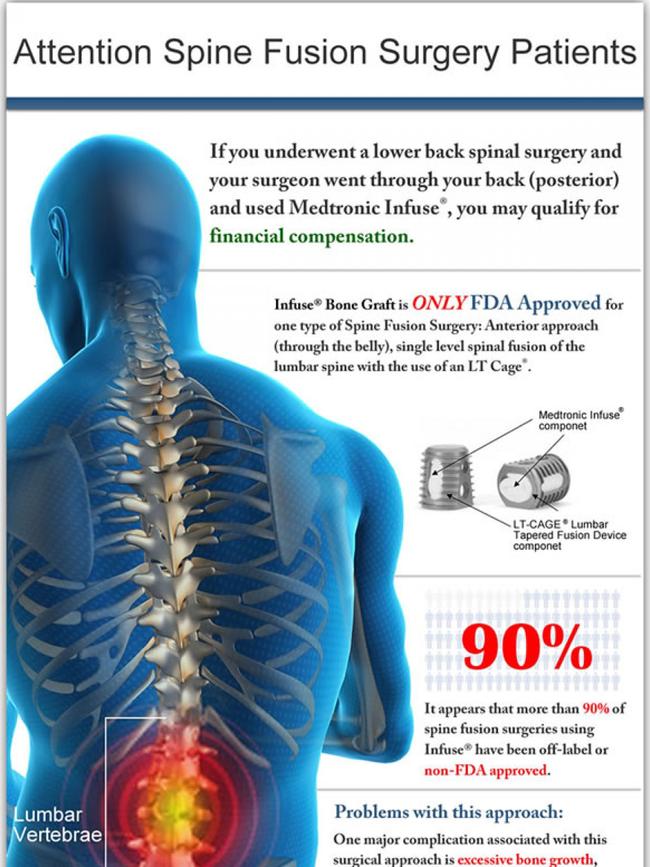
The product, a protein that stimulates the growth of human bone, is used in spinal surgery but
the TGA and the company’s product information sheet state it must be used with Medtronic’s metallic cage.
It is used to treat back pain caused by arthritis or degenerative spinal problems.
However, a whistleblower has revealed it is being widely used off label in Australia and it is almost never used with the Medtronic cage meant to contain it.

Ten adverse events connected to the product have been reported in Australia including:
• abnormal bone formation;
• nerve root compression causing leg pain;
• extreme pain in the left foot;
• and unremitting back pain and left leg pain.
Reviews in the US also found other side effects including inflammation in nearby tissues and bone, urinary problems and even cancer.
In the US 10,000 people sued for the problems it caused them and the company had to pay out $460 million in settlement payouts.
It emerged the company was paying kickbacks of up to $23 million to US doctors as an incentive to use Infuse.
Private Healthcare Australia chief Dr Rachel David said after a whistleblower warned the product was not being used in line with TGA approval, health funds checked and found it was mostly being used without the cage.
“When it’s used off label there must be patient consent,” said Dr David who has written to funds suggesting they may want to reconsider funding the device unless surgeons can produce written consent from patients to the off label use of the product.
The TGA told News Corp that off label use of the product “is a clinical decision and it is a matter for the treating physician to discuss with the patient”.
“It is important that both patients and clinicians who use medical devices off-label are aware of the potential harms, understand that there is no regulatory oversight on these occasions and have undertaken their own risk assessment,” the medical regulator said.
Spine Society Australia’s Associate Professor Matthew Scott-Young said the product had been used in Australia without any problems for 15 years and it was listed on the Prostheses List “without limitation in relation to surgical approach, level of the spine, associated cage or implant or clinical indication”.
“This attack (on Infuse Bone Graft) is without cause and notice and is a fishing expedition to stop funds paying for expensive devices. They want to increase fund profits,” he said.
Doctors in Australia using the Infuse Bone Graft product were using alternative cages to the one supplied by Medtronic which had been superseded, he said.
Asked to explain the huge number of legal cases in the US he said it was a “litigious society” and it might be “the surgeon blaming the device and not his technique”.
MORE NEWS:
Aussie designers stun in New York
Nationals trip ‘coincided with Melbourne Cup’
Students forced to pay study loans sooner
Royals to visit our bushfire towns
Medtronic said: “Patient safety is our top priority. Our policies and training expressly provide that we promote our products only for those uses that are consistent with the labelling approved by the TGA”.
In relation to claims the product was being used off label Medtronic said “the way in which the device is used is a clinical decision made by the surgeon based on patient anatomy, pathology, and treatment plan without Medtronic’s involvement and in the best interests of their patient”.
If you have an experience with Medtronic’s Infuse Bone Graft you wish to share please contact sue.dunlevy@news.com.au
Originally published as Health funds query a cure for back pain subject to lawsuits


