Volunteers may be infected to test COVID-19 vaccines
If the outbreak is brought under control in Britain, a research chief claims healthy volunteers would have to be deliberately infected.
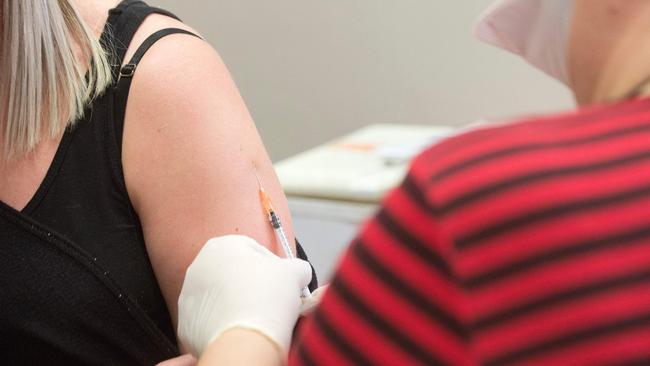
Deliberately infecting healthy volunteers will be the only way to test a coronavirus vaccine in Britain if the outbreak is brought under control, a research chief has said.
Sir Terence Stephenson, chairman of the Health Research Authority (HRA), said that its research ethics committees would consider any such proposal.
The HRA, which approves research undertaken on NHS patients in England, has approved 113 COVID-19 research trials on an accelerated schedule since the pandemic began. It usually considers requests for two months but has been able to give studies the go-ahead after as little as 24 hours.
They include a trial of the Oxford vaccine, based on a virus found in chimpanzees, which is seeking to establish whether it can prompt an immune response in healthy volunteers’ bodies without causing harm.
If a vaccine proves successful in such early trials, it will move forward to studies designed to test its effectiveness in stopping cases of the virus.
Sir Terence said that there were broadly three ways that such a trial might be run. “If we still have a lot of coronavirus infections happening in the UK, then the study could be done in the UK,” he said. “You give half the people the vaccine, half the people a placebo or a different vaccine, and you look and see if the group given the vaccine gets protected from infection.
“That would be the most standard, straightforward way, and no real ethical issue with that.”
Should the UK be in the position of “not having much coronavirus infection”, a second option would be studying it overseas, he said.
“The third possibility, and probably the most contentious, that would have to come to a research ethics committee, is the idea of giving some people the vaccine and then deliberately exposing them to the virus,” he said. “Presumably, if you were to do that you would choose healthy young people with no other conditions, but it wouldn’t be without risk. It’s certainly not for me to give a judgment on whether that would be approved or not: giving ethics approval does not sit with the chairman. But my sense, having talked to people in the field and to some people on the research ethics committee, is that that is something they would have to look at very carefully.
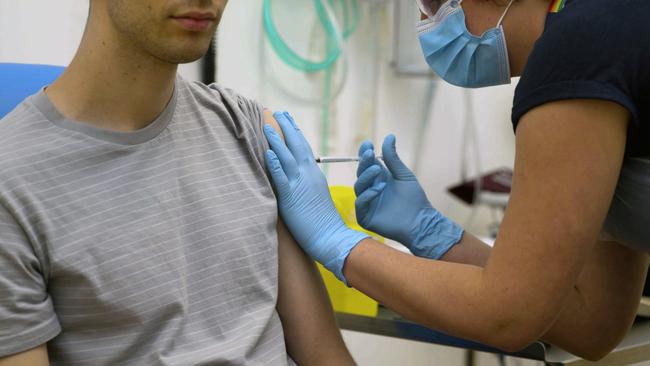
He added: “It is not a decision that has been made yet because it hasn’t come to us, but it is a decision that would be made by one of our research ethics committees.”
About 47,000 people have been recruited to 113 trials approved by the HRA. Other research that has received speedy approval, Sir Terence said, included trials of the antiviral remdesivir and the antimalarial drug hydroxychloroquine for coronavirus patients.
A swifter turnaround for research requests was partly borne of necessity, he said, but the organisation also found that embracing new technology made its processes more efficient. “All of these studies need the ethics of them to be looked at to give approval. You have to convene a group of experts and lay people together in one place.
“So traditionally a face-to-face meeting, perhaps a dozen people, sent the papers in advance. They print them and read them, they meet in London or Newcastle or Oxford and they discuss it and then they write back with their view. All of that is being compressed, everything is sent out electronically. Nobody has to travel anywhere; the meetings are being done by Zoom or other platforms. So we’re able to compress this to achieve this really fast turnaround time.”
He said that the number of requests that the HRA was being called on to approve had reduced “partly because patients aren’t coming into a hospital, and not being recruited”, but also because “many people in universities and scientists have turned their attention to Covid”.
Sir Terence said that the organisation’s COVID-19 experience might inform how it worked in the future, with a move to approving research in order of urgency, rather than its usual “cab rank” system of dealing with applications in the order they were received.
The Times





To join the conversation, please log in. Don't have an account? Register
Join the conversation, you are commenting as Logout