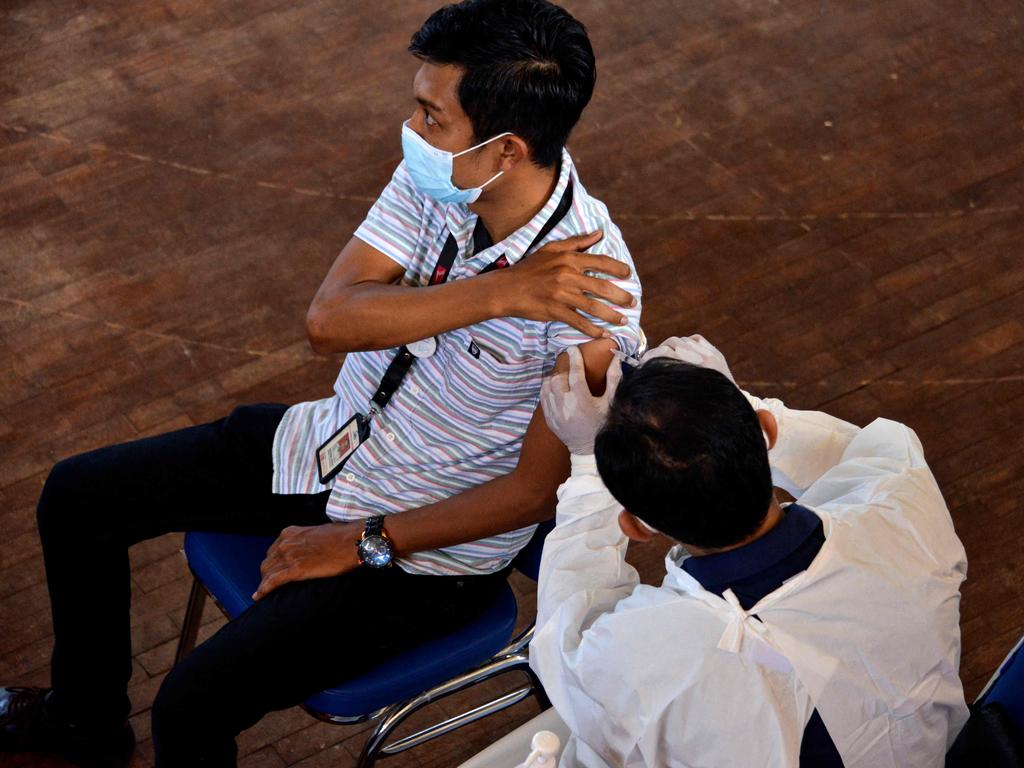
First volunteers given jabs for Beta variant
The first volunteers have received a modified vaccine tailored to combat the Covid-19 variant first identified in South Africa.
The first volunteers have received a booster shot of a tweaked Oxford vaccine tailored to combat the Covid-19 variant first identified in South Africa.
The B.1.351 or Beta variant has been the most worrying in terms of its potential for evading vaccines.
The trial, which will include 2,250 people in the UK and abroad, will test how vaccines can be adapted to combat it. Most of those in the trial will have received either two doses of the Oxford-AstraZeneca vaccine or one of the mRNA vaccines.
Professor Sir Andrew Pollard, director of the Oxford Vaccine Group, said that the trial “is important to ensure we are best prepared to stay ahead of the pandemic coronavirus, should their use be needed”.
The Beta variant, which fuelled a wave in South Africa during the southern hemisphere’s summer, has mutations on its spike protein that mean antibodies targeting the original Wuhan strain are less likely to latch on to it.
It appears to be particularly good at blunting the Oxford vaccine, with one small trial suggesting it had only minimal efficacy at stopping infection, if not severe disease. Some immunologists believe, though, that it could also be the best target for vaccines, as it appears to offer “backwards compatible” immunity, protecting strongly against other variants too.
The trial is far smaller than those that tested the original vaccines. It is believed that regulators will only require data on immune response and safety for updated vaccines, rather than the full efficacy results needed for the first generation, which required trials involving tens of thousands of people.
This means that the scientists will not have to wait for enough cases in the trial before reporting results. Even so, the booster will not be ready for the autumn. It is expected that those receiving a third shot before winter will be given the original vaccine formulation.
Sir Mene Pangalos, from AstraZeneca, said that the work is a way of “staying ahead of genetically distinct variants. “Initiating the [trial] means we can be prepared should a variant vaccine be required in the future.”
The Times