Russian COVID-19 vaccine, Sputnik V, ‘92 per cent effective’
Moscow confident inoculations will begin in weeks as data suggests ‘Sputnik V’ has similar efficacy levels to Pfizer vaccine.
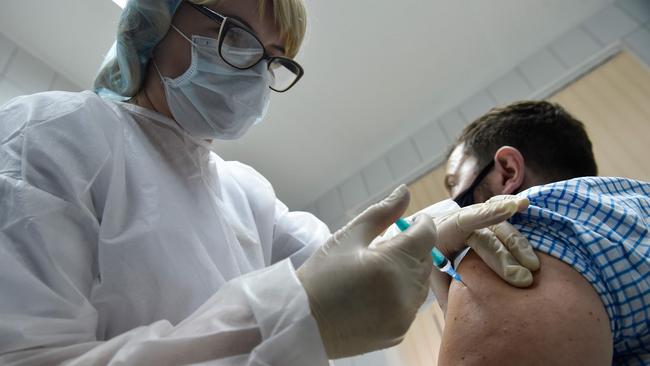
New data suggests Russia’s coronavirus vaccine, Sputnik V, is 92 per cent effective, raising hopes the development of more global vaccines will help stem the coronavirus pandemic.
The efficacy of the Sputnik V vaccine is similar to the 90 per cent efficacy levels registered by the Pfizer BioNTech vaccine, but it involved fewer positive coronavirus cases upon which to calculate the data.
Australia has not committed to purchase any of the Sputnik V vaccine, but the results have given further heart to global researchers that multiple vaccines will be approved by early next year.
Covid-19 news:
— New Scientist (@newscientist) November 11, 2020
• EU to buy up to 300 million doses of Pfizer-BioNTech vaccine candidate
• Russia says Sputnik V vaccine candidate is 92 per cent effective
• US records more than a million new coronavirus cases in first 10 days of November
https://t.co/KnlrBiWwKn pic.twitter.com/wxmTj9UyJW
Australia has committed to up to five different vaccine types if they prove to be safe and effective: purchasing 10 million doses of the Pfizer BioNTech vaccine, 3.8 million doses of the Oxford vaccine (and then the right to manufacture 30m doses of it within Australia), 51 million doses of the University of Queensland CSL vaccine, 40 million doses of Novavax, and a global share of the Covax Facility which incorporates most of the major vaccine developers.
The latest Sputnik V results released on Wednesday, which have not yet been peer-reviewed, come from the first interim data from double-blind, randomised, placebo-controlled Phase III clinical trials in Russia, Belarus, United Arab Emirates, Venezuela and India, involving 40,000 volunteers. During the Sputnik V trials 20 people contracted coronavirus, most of whom had been given the placebo.
In a release issued by the Gamaleya Centre — part of the Russian Ministry of Health — the Sputnik trials evaluated efficacy among 16,000 volunteers who have received the second dose of the vaccine or placebo 21 days after the first injection.
#Breaking
— Geeta Mohan گیتا موÛÙ† गीता मोहन (@Geeta_Mohan) November 11, 2020
The first interim data analysis of the #SputnikV vaccine against covid 19 phase 3 clinical trials in the Russian federation demonstrated 92 percent efficacy--The Gamaleya National Research Center @rdif_press @MoHFW_INDIA pic.twitter.com/WTyQIU0FQT
“As a result of a statistical analysis of 20 confirmed cases of coronavirus, the case split between vaccinated individuals and those who received the placebo indicates that the Sputnik V vaccine had an efficacy rate of 92 per cent after the second dose,’’ the Gamaleya centre said.
This was in addition to vaccination of 10,000 volunteers from Russian hospitals which produced similar results, it added.
The Pfizer BioNTech results showed 90 per cent efficacy after 94 people out of nearly 40,000 volunteers, most of whom received the placebo, contracted coronavirus.
Unlike the Pfizer BioNTech method, which uses messenger RNA, the Sputnik V vaccine uses a more traditional human adenoviral vector platform and doesn’t require deep freeze storage conditions.
There were no unexpected adverse events during the Sputnik V trials, the researchers said. Some side effects of the vaccination were pain at the injection site, flu-like syndrome including fever, weakness, fatigue, and headache.
“The use of the vaccine and the results of clinical trials demonstrate that it is an efficient solution to stop the spread of coronavirus infection, а preventive healthcare tool, and this is the most successful path to defeat the pandemic,” Russian health minister Mikhail Murashko said.
Mass vaccination would begin in Russia in the coming weeks, Gamaleya director
Alexander Gintsburg said.
He said production capacity at new manufacturing sites would mean the Sputnik V vaccine will be available to a wider population, first in Russia and then globally.
Other vaccine trials are expected to announce stage three results within the next few weeks, including the Moderna trials and the Oxford AstraZeneca trials.




To join the conversation, please log in. Don't have an account? Register
Join the conversation, you are commenting as Logout