Coronavirus: ‘Millions of vaccine doses’ coming
Drug-maker CSL will produce millions of doses of Australia’s experimental COVID-19 vaccine as it is being tested to speed up an anticipated rollout.
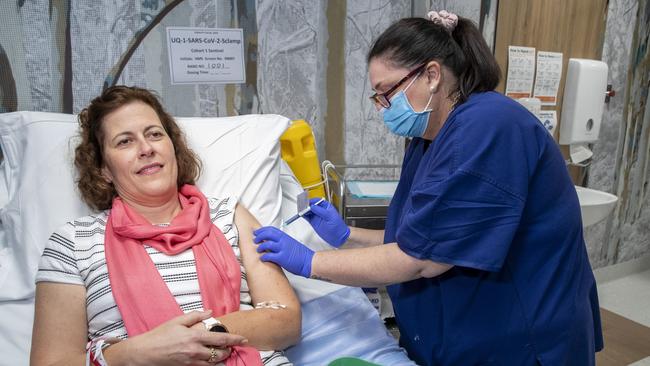
Drug-maker CSL will produce millions of doses of Australia’s experimental COVID-19 vaccine as it is being tested this year to speed up an anticipated rollout.
The company is in discussions to produce “hundreds of millions” doses of the drug in 2021, assuming clinical trials are successful and it is approved by Australian and international regulators.
The decision by the country’s most valuable listed company to push the button on manufacturing came as the vaccine was given to people for the first time in phase-I clinical testing. The University of Queensland scientists behind it said on Monday the immuniser could be ready for emergency use in the new year.
With Premier Annastacia Palaszczuk looking on, the first of 120 volunteers was injected to prove the drug is safe and effective.
CSL, which is partnering with UQ to fast-track the vaccine, will make “several million” antigen doses before the human trials are completed, punting on the prototype vaccine passing the three-stage proving process and being licensed.
“A portion of these (doses) will be used in phase II/III clinical studies, with the remainder available as soon as marketing authorisation is granted,” a company spokesman said.
“We anticipate producing tens of millions of doses during 2021, which would be made available subject to safety and efficacy data generated by trials as well as regulatory approval.
“Initially, CSL will manufacture vaccine from its biotech manufacturing facility at Broadmeadows. Further scale-up of production to hundreds of millions of doses will be achieved in partnership with a contract manufacturer.
“To this end, we are in early discussion with global manufacturers regarding production.”
Ms Palaszczuk emphasised the importance of the vaccine being made in Australia.
“That has always been the missing piece … we have to get overseas companies to do that production,” she said. “(This time) it can be made here locally and distributed internationally.”
She said this would be worth “millions if not billions” of dollars to the nation.
UQ project leader Trent Munro said the scientists had been working around the clock to meet “aggressive timelines” to ready the vaccine to be tested on people, a critical phase of its development.
Preclinical trials on laboratory animals in May showed it delivered higher immunity levels to COVID-19 than those in people who had recovered from the disease.
Yet many new drugs fail in human testing and Professor Munro said there was no guarantee the UQ vaccine would get through. “I think it’s still early days, but everything we have seen gives us confidence that we should keep moving forward,” he said.
“We feel really positive … all the data we have seen is good but like any vaccine trial, this may or may not succeed.”
Work that would normally take years to bring a new drug to clinical trial has been crammed into barely five months since the UQ team adapted its cutting-edge molecular clamp vaccine technology to COVID-19.
Being protein-based, the candidate vaccine is considered safer to test on people than formulations using live virus. The volunteers, ranging from 18 to 55, will be given two treatments four weeks apart in a randomised, double-blind, placebo-controlled study.
Another lead researcher, Paul Young, said the aim was to have the vaccine mass-produced and widely available by mid-2021.
The drug could be released as early as January for emergency use if it performed to expectation in the human trials. Nursing homes hit by a coronavirus outbreak or situated in an area where the disease was surging would be considered for such use, public health experts say.
Only a handful of the 180-odd vaccines in development around the world are in human trials, putting the promising UQ offering in the top tier of contenders from the US, Britain and China.
In the US, an RNA vaccine developed by Boston-based biotech Moderna Therapeutics completed successful phase-I human trials in May and is due to go to phase-II/III testing by the end of the month. Other US companies on the trail include pharmaceutical giants Pfizer and Johnson & Johnson; Pennsylvania-based Inovio has also reported strong immune responses in phase-1 subjects of its DNA-based vaccine.
Britain’s Oxford University group has human trials under way, as do Brazil and South Africa. China’s CanSino Biologic has been cleared by Beijing to test its potential vaccine on the country’s two million-strong army.
Professor Young said rival research teams were not competing, and the world would need more than one vaccine.








To join the conversation, please log in. Don't have an account? Register
Join the conversation, you are commenting as Logout