Coronavirus: Anti-malarial drug trials abandoned
Scientists leading a clinical trial have abandoned testing controversial antimalarial drug hydroxychloroquine.
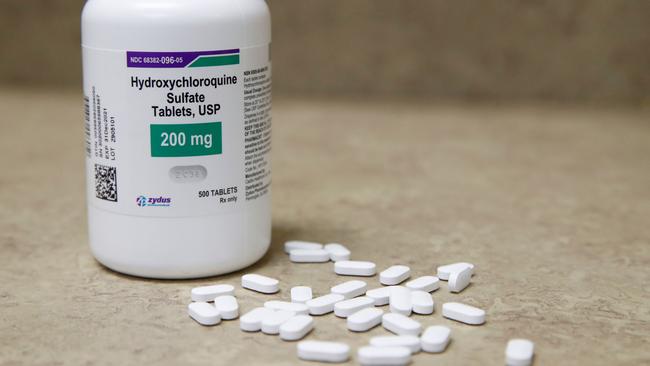
Scientists leading a clinical trial that aims to prevent coronavirus patients needing intensive care have abandoned testing antimalarial drug hydroxychloroquine on the basis that it is not effective in treating COVID-19.
Instead, the scientists will administer convalescent plasma to COVID-19 patients in a recasting of the clinical trial.
Melbourne’s Doherty Institute is conducting a major randomised control trial known as the ASCOT trial, set to enrol more than 2000 patients in hospitals in Australia and internationally. Previously, the trial was testing the efficacy of hydroxychloroquine and anti-HIV drug lopinavir, given either separately or in combination.
The leaders of the ASCOT study made the decision to discontinue administering hydroxychloroquine and lopinavir after a major clinical trial by Oxford University in Britain in conjunction with the World Health Organisation found the two drugs were not effective in reducing COVID-19 mortality.
“We have made this decision based on the reports of the UK study, plus growing evidence from several smaller studies showing hydroxychloroquine and lopinavir are not effective in treating COVID-19,” said ASCOT principal investigator Steven Tong, a Royal Melbourne Hospital infectious diseases clinician and co-lead of clinical research at the Doherty Institute.
Professor Tong said antiviral drug remdesivir had been shown to be more effective in reducing the severity of COVID-19, together with anti-inflammatory drug dexamethasone.
Most patients in hospital with severe COVID-19 were receiving these treatments, he said.
The effectiveness of convalescent plasma in treating COVID-19 has not been established in major clinical trials, but Professor Tong said its use during pandemics had a long history.
“Convalescent plasma has been used historically for the 1918 Spanish and the 2009 influenza pandemics, for pneumococcal pneumonia, and also for previous coronaviruses — SARS and MERS,” he said.
“Over 20,000 patients in the US have safely received convalescent plasma for COVID-19. While conceptually attractive, we still need clinical trials to demonstrate efficacy for patients with COVID-19.”
As part of the immune response, people recovering from COVID-19 can develop antibodies targeting parts of the SARS-CoV-2 virus. These antibodies are contained in the liquid part of the blood, the plasma, and can be given to patients newly infected with COVID-19 via plasma transfusion, potentially resulting in more rapid control and clearance of the virus.
Red Cross Lifeblood is collecting convalescent plasma from recovered coronavirus patients. It’s already received more than 1000 donations from 474 donors who have recovered from COVID-19.
There is a smaller clinical trial of convalescent plasma under way in Australia, led by pharmaceutical company CSL. CSL is also working on a hyper-immune COVID-19 immunoglobulin product made from purified and concentrated antibodies.
Convalescent plasma will also be given to patients in a second clinical trial led by the Doherty Institute and Monash University, called REMAP-CAP.
“The convalescent plasma arms of these trials complement each other,” said Monash University associate professor Zoe McQuilten.




To join the conversation, please log in. Don't have an account? Register
Join the conversation, you are commenting as Logout