Coronavirus explainer: Latest about AstraZeneca vaccine and blood clots: your questions answered
The small number of cases observed after the AstraZeneca shot are not ordinary clots. How significant is the risk?
Extremely rare cases of blood clots have been observed in people throughout the UK and Europe following vaccination with the AstraZeneca shot. But these are no ordinary blood clots — they are of a type that doctors have rarely seen before. After the European medicines regulator found the clots were a very rare side effect of the AstraZeneca vaccine, Australia recommended against the use of the jab in those aged under 50.
What is this blood clotting syndrome and how significant is the risk?
The blood clotting syndrome has been dubbed vaccine-induced prothrombotic immune thrombocytopenia (VIPITS) and the European Medicines Agency says it has occurred at the rate of roughly one in 100,000 people who receive the AstraZeneca vaccine, It’s been seen mostly in people aged under 55 and is three times as likely to occur in young women than men. In comparison, the risk of blood clots for women taking the oral contraceptive pill is around 40 in 100,000. Australia has so far seen one case, in a 44-year-old Melbourne man.
Most of the cases of VIPITS have manifested in cerebral clots in the brain, known as cerebral venous sinus thrombosis (CVST). It can also cause blood clots in the abdomen known as splanchnic vein thrombosis. It is difficult to predict who might suffer the vanishingly rare condition as pre-existing health conditions do not appear to influence a person’s risk, and neither is there a link with the contraceptive pill.
VIPITS, that occurs between four and 20 days after vaccination, is characterised by clotting with a low blood platelet count, known as thrombocytopenia.. It is thought that in these very rare cases, an antibody reaction by the body to the vaccine induces massive platelet activation, reducing the platelet count and causing thrombosis.
What has the European Medicines Agency said?
The EMA received reports of 169 cases of CVST and 53 cases of splanchnic vein thrombosis. The EMA has concluded that these clotting disorders are “very rare side effects” of the AstraZeneca vaccine, but said it could identify a definite cause for the complications. It has recommended these conditions should be listed as possible side effects of the vaccine on the product label. The EMA says that on balance, the benefits of the vaccine continue to outweigh the very small risks.
What has Australia’s reaction been?
The Australian Technical Advisory Group on Immunisation has recommended that the Pfizer vaccine is now preferred over use of the AstraZeneca vaccine in those aged under 50. Australia’s chief medical officer Paul Kelly says the AstraZeneca vaccine is very safe for those aged over 50 and the risk of developing the blood clotting syndrome is extremely remote. It’s advised that those aged under 50 who have already had one AstraZeneca jab receive their second shot.
What steps have other countries taken?
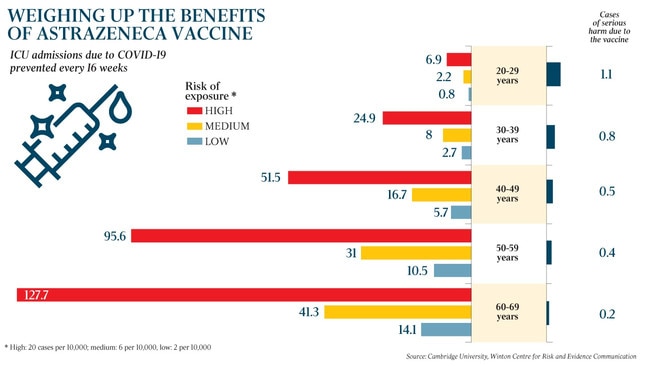
The UK has announced it will give an alternative vaccine to people aged under 30. It says the risk of entering ICU in a low-exposure environment for someone in their twenties is 0.8 per 100,000 people, versus a serious harm from the AstraZeneca vaccine of 1.1 cases per 100,000 people. The risk to benefit ratio therefore falls in favour of restricting the use of the vaccine in young people. Many European countries, as well as Canada, have restricted the vaccine to those aged under 55.
What options does Australia have for vaccines for younger people?
Australia has 20 million doses of Pfizer vaccine so that vaccine will now be reserved for those aged under 50. Australia is also attempting to secure more Pfizer. Australia also has orders for 51 million doses of the the Novavax jab, which will become available later in the year, so young people may be able to receive these vaccines instead.
More Coverage
Coronavirus explainer: Latest about AstraZeneca vaccine and blood clots: your questions answered





To join the conversation, please log in. Don't have an account? Register
Join the conversation, you are commenting as Logout