Coronavirus: Blood clot fears force AstraZeneca switch
Australians aged under 50 will not be given the AstraZeneca vaccine under advice from the nation’s health advisers.

Australians aged under 50 will not be given the AstraZeneca vaccine under recommendations from the nation’s health advisers that have cast doubt on plans to give the whole population a jab by October.
Scott Morrison on Thursday night said a major “recalibration’’ would be required to the vaccination program for under-50s, with the government forced to rely on the imported Pfizer vaccine or the yet-to-be-approved Novavax to complete the program.
Responding to the findings of the European Medicines Agency that there was a link between the AstraZeneca dose and a rare blood-clotting syndrome, the Australian Technical Advisory Group on Immunisation recommended the Pfizer vaccine now be given in preference to AstraZeneca for people aged under 50.
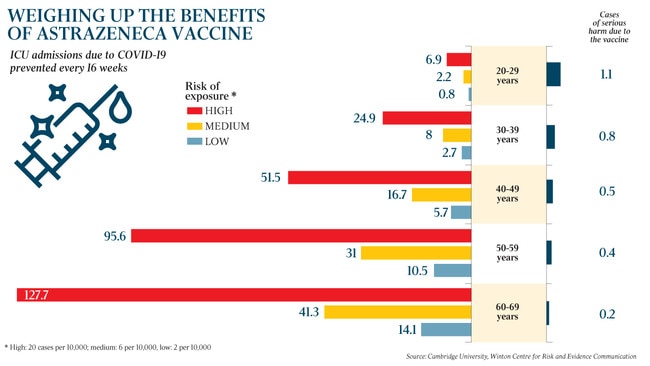
ATAGI advised that the AstraZeneca vaccine, which is made locally, was safe for those aged over 50, and said those in priority groups aged under 50 who had already been given one AstraZeneca shot could safely receive the second jab.

Mr Morrison said ATAGI had issued the new advisory “out of an abundance of caution” in light of the lower risks of becoming seriously ill and dying from COVID in the younger age groups and given the low incidence of COVID-19 in Australia.
“We’ve been taking the necessary precautions based on the best possible medical advice,” Mr Morrison said. “We have always taken the time to ensure we get that advice, consider it carefully, and make decisions in the best interests of Australians and those best interests, principally, have to address the health of Australians.”
The government is encouraging people to consult with their doctor before getting a vaccine and said there was no restriction on use of the AstraZeneca shot in those aged under 50 should people deem it to be in their own interest.
“There is not a prohibition on the use of the AstraZeneca vaccine for persons under 50,” Mr Morrison said. “There is an expression of a preference.”
Federal Health Department secretary Brendan Murphy said the decision was taken despite the blood-clotting syndrome being extremely rare.
“This is a very, very rare event, and it is a highly precautionary position that Australia can take because we’re in a fortunate position with COVID,” Professor Murphy said.
“All vaccines have adverse effects, some serious. But this syndrome, after all of the work we’ve done with the UK and Europe, does seem to be a real syndrome, and we now feel that out of an abundance of caution, given that this syndrome seems to occur mainly in younger people for whom the risk of severe COVID is not so great, that there is a basis to have a preferred recommendation for those under 50s.
“We are strongly encouraging those 50 and over to take up the AstraZeneca vaccine. It is a highly effective vaccine at preventing severe COVID. The risk is extraordinarily low.”
Australia is urgently seeking to purchase more Pfizer vaccine doses to ensure there is enough coverage for the younger population. Pfizer will be fully reserved for younger groups and it’s not expected that there will be any delay to the first two phases — 1A and 1B — of Australia’s vaccination program. But vaccinating the whole population will now be pushed well into 2022, almost certainly scuttling plans to reopen borders and the economy later this year.
Australia has ordered 20 million doses of Pfizer but it is coming piecemeal and the full 20 million shots will not be all received until the end of the year. Australia has also ordered 51 million shots of the Novavax vaccine which could be given to the young, but it won’t be available until the third quarter of this year. CSL may be able to manufacture that vaccine onshore, but not while they are making AstraZeneca.
“We expect that this recommendation will require some changes to the arrangements we have as part of the vaccination rollout,” Mr Morrison said.
“And this includes when we might expect our first doses, ultimately, to be able to be offered to all Australians”
Australia’s decision to advise against the use of the AstraZeneca vaccine in those aged under 50 goes much further than the UK’s decision to offer an alternative vaccine to those under 30. But it is in line with many European countries, including France, Germany and Italy, as well as Canada.
The European Medicines Agency found that blood clots associated with low platelet counts were likely a rare side effect of the AstraZeneca vaccine.
The EMA, which examined more than 100 cases of what has been dubbed vaccine-induced prothrombotic immune thrombocytopenia (VIPITS), said the blood clots had occurred in approximately one in every 100,000 people who had been vaccinated in Europe. The UK has seen a lower incidence at one in 250,000. One case of VIPITS has so far occurred in Australia, in a 44-year-old Melbourne man who received the AstraZeneca vaccine in late March.
VIPITS is a very unusual blood clotting syndrome as it is characterised by clotting with a low blood platelet count, known as thrombocytopenia.. The exact mechanism is unclear, but it is thought that the vaccine induces an antibody reaction that over-activates the platelets in the blood, reducing the platelet count and causing thrombosis.
Most of the cases recorded have been brain clots known as cerebral venous sinus thrombosis (CVST), but there have also been cases of an abdominal clotting syndrome called splanchnic vein thrombosis. The clotting occurs between four and 20 days after vaccination.
Following an intensive investigation, the EMA’s Pharmacovigilance Risk Assessment Committee concluded that the reported cases of unusual clotting after receiving a dose of the AstraZeneca vaccine should be listed as a possible side effect of the jab.
A total of 169 cases of CVST have been reported following vaccination in Europe out of 34 million people who received the AstraZeneca jab. There were 53 cases of splanchnic vein thrombosis.
“Our conclusion is that these clotting disorders are very rare side effects of the vaccine,” said EMA safety committee chairwoman Sabine Straus.
“Current available data did not allow us to identify a definite cause for these complications. However possible plausible explanations have been put forward, including an immune response that leads to a condition that seems similar to atypical to heparin-induced thrombocytopenia.”
EMA executive director Emer Cooke said the regulator was not able to draw any link between the risk of VIPITS and age or gender — even though most of the cases had been in young women.
Taking the oral contraceptive pill was not linked with a greater risk of vaccine-induced clotting, she said.
“No specific risk factors could be identified based on the current data,” Ms Cooke said.
“The committee can therefore not recommend any specific measures to reduce the risk.”




To join the conversation, please log in. Don't have an account? Register
Join the conversation, you are commenting as Logout