Flinders University to sever ties with ‘refusenik’ vax developer Nikolai Petrovsky
Vaccine developer Nikolai Petrovsky is set to lose of his academic affiliation at Flinders University after a meeting of its senior executive resolved to end its relationship with the professor.

Vaccine developer Nikolai Petrovsky is set to lose his academic affiliation at Flinders University after a meeting late last year of its senior executive resolved to end its relationship with the professor.
The meeting in mid-November of the university’s senior executive, involving six of its foremost academics and administrators, moved to end Professor Petrovsky’s affiliation with Flinders University, following a review that considered his teaching and research contributions to the Adelaide-based institution.
The review, which included hundreds of other academic affiliates, assessed whether Professor Petrovsky had “a regular (and) significant academic involvement” in teaching and research at Flinders, as well as an “involvement which entails significant academic leadership”.
In response to questions, a university spokesman said Professor Petrovsky’s academic status was in the “process of being finalised – as such, the university will not make any comment that could prejudice the process”.
The Australian understands Professor Petrovsky engaged lawyers to challenge the senior executive’s decision late last year, as well as the terms of his departure, a negotiation that is ongoing.

Professor Petrovsky said he was “not prepared to discuss matters relating to legal representation”, adding that he continued to enjoy “academic status at Flinders University”. A university source, with knowledge of the affiliate review process, said Professor Petrovsky would have been treated like any other applicant who was required to reapply for their affiliate status.
“His commitment is to his company (Vaxine Pty Ltd) and that’s very, very clear,” said the university source, who wished to remain anonymous.
“It’s an entirely legitimate mechanism for doing research or for publishing research, but it just didn’t relate to Flinders other than he was using our space and our affiliate [professor] title.”
The scientist made national headlines in 2021-22 after the development of his protein-based vaccine Covax-19, and his refusal to get jabbed with an approved Covid vaccine.
Professor Petrovsky later faced dismissal from Adelaide’s Flinders Medical Centre, where he remains director of endocrinology, in late 2021 for rejecting SA’s vaccine mandate.

At the time he told The Australian it would be “dangerous” and potentially “counter-productive” to his health to be subjected to another round of vaccination after he had administered himself with his Covax-19 formula.
“There is no clinical data on what would happen if someone who is fully vaccinated subjected themselves to a whole further round just to satisfy an arbitrary mandate. It would not be safe,” he said in November 2021.
It was later revealed Professor Petrovsky had previously written to participants of his Phase 1 clinical trial, encouraging them to take a vaccine approved by the Therapeutics Goods Administration when it became available, “just as you would have, had you not participated in this study”.
After several participants contacted the Central Adelaide Local Health Network questioning the professor’s public statements, the research ethics committee’s acting chair, David Evans, instructed participants to ignore Professor Petrovsky’s claims in the media and get a TGA-approved vaccine.
“Participants are concerned that comments that Professor Nikolai Petrovsky reported in the media did not correspond with the advice given in the letter participants received,” Dr Evans wrote in November 2021.
“These comments … could be construed that it would be unsafe to receive a TGA-approved vaccine … The committee is not aware of any scientific evidence of efficacy of the Covax-19 vaccine to meet Australian TGA standards.”
Phase 1 clinical trials are primarily conducted to test whether a vaccine is safe. However, the Covax-19 Phase 1 trial also examined the “immunogenicity” of the vaccine, or whether it produced an immune response.
Professor Petrovsky attracted heavy criticism in some scientific quarters for comments regarding the safety and efficacy of mRNA vaccines after he described them as experimental as well as “gene therapy vaccines” – a claim rejected by the TGA.
In April, Vaxine was fined $13,320 by the TGA after the regulator said it had “allegedly advertised … an unapproved Covid-19 vaccine which is subject to a clinical trial”.
In December, the provisional determination status granted to Vaxine by the regulator lapsed after no further clinical data was supplied. The Australian understands the TGA has not received any application to register the product.
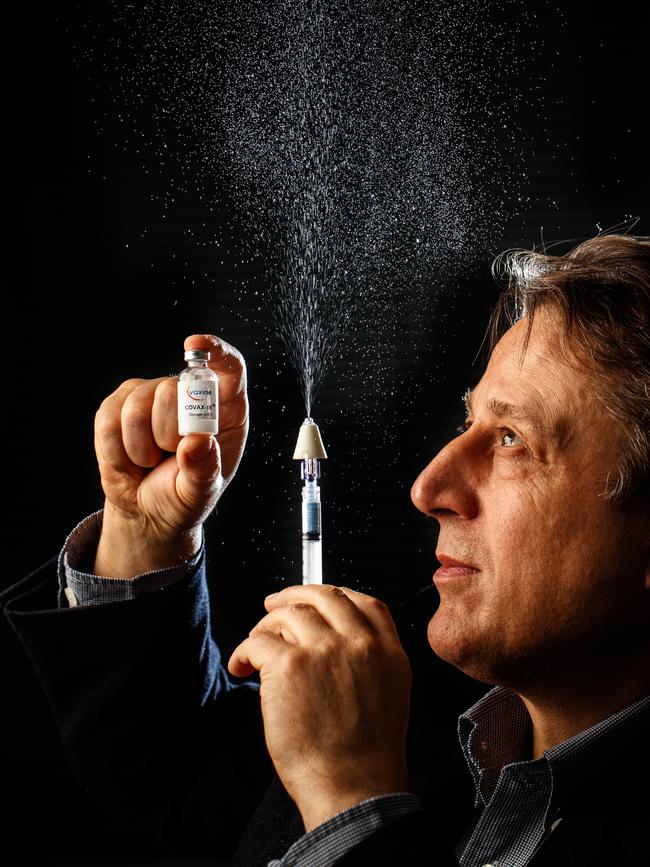
Professor Petrovsky maintains millions of doses of Covax-19 have been administered in Iran, under the name SpikoGen, where he says he has successfully completed Phase 2 and 3 clinical trials.
The company’s Phase 2B clinical trial — the second to be conducted in Australia since Vaxine started a Phase 1 study in 2020 — was launched in collaboration with the Australian Respiratory and Sleep Medicine Institute in Adelaide last year and is due to be completed in March.
In response to questions, a Flinders University spokesman said the university conducts regular reviews of academic status for eligibility in accordance with university policy, but would not comment on Professor Petrovsky’s affiliation process.
“As status holders’ terms near expiry they are invited to apply for renewal as per normal college processes. In 2022 this comprised several hundred applications which were assessed in accordance with the current University Academic Status policy,” the spokesman said.






To join the conversation, please log in. Don't have an account? Register
Join the conversation, you are commenting as Logout