Second trial for unproven Australian Covid-19 vaccine
The developer of a controversial Australian coronavirus vaccine will hold a second clinical trial, as he seeks regulatory approval for his product.
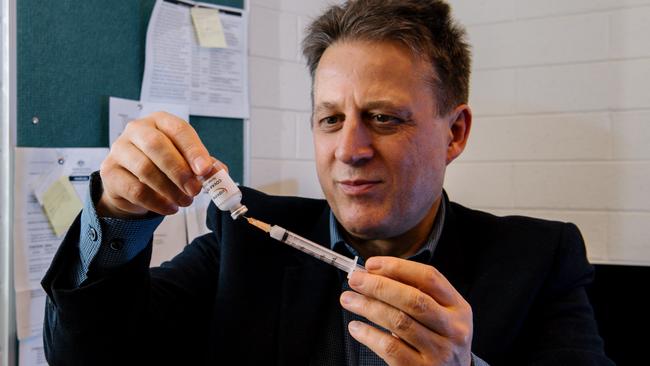
Controversial vaccine developer Nikolai Petrovsky has launched a second clinical trial seeking regulatory approval for his coronavirus vaccine in Australia, after recruiting 200 participants for a clinical trial in Adelaide.
Professor Petrovsky’s Phase 2B clinical trial – the second to be conducted in Australia since he started a Phase 1 study of Covax-19 in 2020 – has been launched in collaboration with the Australian Respiratory and Sleep Medicine Institute in Adelaide and will test the effect of “varying the time interval between doses” on the vaccine’s effectiveness.
It comes less than a year after the Phase I Human Research Ethics Committee told study participants there was no scientific evidence to support its efficacy to Therapeutic Goods Administration standards.
Last week Professor Petrovsky’s company, Vaxine, was slapped with a $13,320 fine by the TGA after the regulator said it had “unlawfully” and “allegedly advertised” on its Facebook and YouTube accounts “an unapproved Covid-19 vaccine which is subject to a clinical trial”.
The regulator said Vaxine had failed to address “a range of concerns” related to its promotion of Covax-19, which TGA officials had raised with Professor Petrovsky via phone and in writing.
“The advertising of unapproved therapeutic goods to the public, including a vaccine undergoing a clinical trial, is not permitted in Australia as those goods have not been assessed by the TGA,” a statement read.
In a short video uploaded to the company’s social media accounts, Professor Petrovsky announced Vaxine had launched its Phase 2B trial of Covax-19, while Sharen Pringle – Vaxine’s business manager and Professor Petrovsky’s wife – appeared to interview one of the trial participants after he had received a vaccine.
Ms Pringle asked the participant why he wanted to be on the trial and whether he thought it was important that Australian-developed vaccines were available to Australians.
The infringement notice comes after The Australian revealed details of Covax-19’s Phase 1 clinical trial, including correspondence from Professor Petrovsky and the acting chair of the Human Research Ethics Committee, in which trial participants were instructed to get vaccinated with a TGA-approved jab because of the “low likelihood of its (Covax-19’s) effectiveness”.
Phase 1 clinical trials are primarily to test whether a vaccine is safe. However, the Covax-19 Phase 1 trial also examined the “immunogenicity” of the vaccine, or whether it promoted an immune response.
In his own letter to Phase I participants, Professor Petrovsky stated: “Antibody responses to the vaccine were low. Immune responses measured by other tests are still pending.”
In a separate letter from the ethics committee’s acting chair, David Evans, participants were told the trial’s investigating committee was “not aware of any scientific evidence of efficacy of the Covax-19 vaccine to meet Australian TGA standards”.
Professor Petrovsky claims six million doses of Covax-19 have now been administered in Iran, under the name SpikoGen, where it has completed Phase 2 and 3 clinical trials. But the absence of any publicly available clinical data to substantiate its effectiveness has raised concern among experts, who have questioned the legitimacy of the Iranian trials.
Since October, Professor Petrovsky company has raised more than $1m in public donations through a GoFundMe site to support its application for TGA approval.







To join the conversation, please log in. Don't have an account? Register
Join the conversation, you are commenting as Logout