With STIs on the rise, Australia’s first rapid tests promise speed, privacy
Women will soon be able to test themselves for one of the most commonly reported communicable disease in Australia as cases surge.

At-home STI tests for chlamydia and gonorrhoea will soon be available in Australia for the first time, intended to give accessible and judgement-free diagnoses amid rocketing infection rates.
While previously barred by the Therapeutic Goods Administration, changes in the wake of the pandemic allowed the development of self-tests for STIs, with the first such option receiving approval this month.
The rapid tests are the first of their kind in Australia, allowing Australian women to test for chlamydia and gonorrhoea through a pharmacist or distributor without prescription.
Both conditions have seen surging case rates in the past decade, with chlamydia and gonorrhoea increasing by 26 per cent and 157 per cent over the period, respectively.
It has brought chlamydia to the point of being the most commonly reported communicable disease in Australia.
They are also commonly mistaken for other conditions, or appear asymptomatic for long enough to result in further infection.
“In Australia, chlamydia and gonorrhoea rates have been increasing in recent years, and we know that early testing, diagnosis, and treatment of STIs prevent serious long-term outcomes, including pelvic inflammatory disease and infertility. Being able to test for chlamydia and gonorrhoea, two common sexually transmitted infections, using a simple vaginal swab rapid test at home is convenient and maximises privacy. This self-test may increase participation in STI testing by reducing barriers, such as stigma, increasing accessibility and comfort, and receiving results faster to take appropriate action,” University of Sydney adolescent health researcher Cristyn Davies said.
“If you are sexually active, regular testing is the only way to check if you have an STI, as you may have an STI and not have symptoms. Being able to test for chlamydia and gonorrhoea yourself may make it easier for you to have the test. Those who receive a positive test must consult their healthcare professional for confirmatory testing and advice regarding treatment.
“Increased testing could lead to the earlier identification and treatment of STIs, which may reduce their transmission rates in the community. By normalising the testing process, individuals may feel more comfortable checking their status regularly, leading to better control of STI prevalence.”
The TGA mandates a minimum threshold of 95 per cent accuracy for self-tests, which the STD tests exceeded, averaging 99 per cent.
Chlamydia and gonorrhoea are notifiable diseases and contact tracing is mandatory. Praise for the approval for the tests was not universally expressed by doctors. Some doctors are concerned that home testing may have the potential to derail the nation’s STI management systems. The TGA only checks sensitivity and specificity of tests, and there are concerns that self-test kits may mean that people diagnosed with STIs will not receive crucial information required to halt transmission.
The health technology was manufactured by Sydney-based pharma developer Touch Biotechnology. Sales director Steven Quinlan said the company expected the bulk of tests to retail online, while reducing burdens on the primary care sector.
“Most clinics don’t have the facilities to (test for STIs) on their premises, so they’ll send it away. So someone with an infection will have to travel to get some medical advice, then provide a sample, then wait for the results, and then go back to the doctors,” Mr Wallace said.
“Giving them this opportunity means they can do the tests in private, get the results quickly, and it detects both (infections) using the same sample.
“What we’re seeing with these self-tests off the back of Covid (is) people are much more familiar with it.”
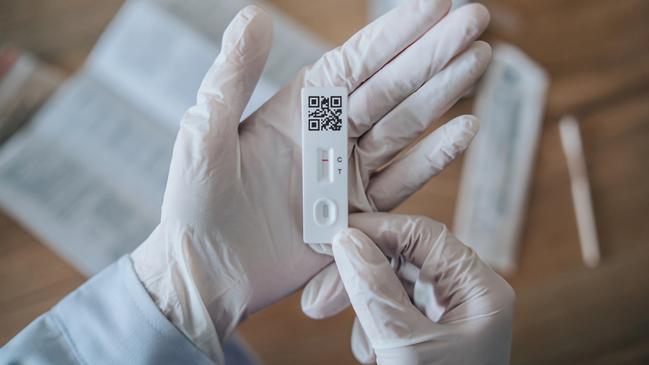
The rapid tests were approved by the TGA on November 11, with a pharmacy rollout ongoing. Once available, they are expected to retail for $24 a unit.
Prior to the TGA listing, gonorrhoea and chlamydia could only be screened in Australia with individual lab or PCR tests. The average wait for results in such tests is one to three days.
If left to complicate, chlamydia and gonorrhoea can inflict pelvic inflammation and infertility.
Touch Biotechnology chief executive Matt Salihi said it was still important to follow up at-home diagnoses with healthcare consultation.
“It’s incredibly important for women, and men, to consider the risks of unprotected sex because despite the perceived stigma, STIs impact so many Australians amounting to much more than uncomfortable symptoms,” Mr Salihi said.
“We hope it will empower women to take the first step towards receiving a diagnosis.”







To join the conversation, please log in. Don't have an account? Register
Join the conversation, you are commenting as Logout