Compliance or defiance? Cosmetic injectables industry in turmoil as authorities crack down
Compliance checks of the cosmetic injectables industry are being conducted, as Queensland grapples with its own newly enforced rules.
Audits of the non-surgical cosmetics industry are under way to check compliance with legislation at both state and federal levels.
The Australian understands the largest of the audits is being undertaken by the Therapeutic Goods Administration, which has been monitoring the websites and social media accounts of practitioners who work in cosmetic injectables.
Around 100 “guidance letters” have been issued to people it believes are in breach of advertising rules. The letters set out the TGA’s concerns and the person’s legal obligations. They are then told to fix any breaches within a time frame.
In Australia it is illegal to advertise almost all prescription medications or the services associated with them. That includes cosmetic injectables such as Botox and dermal filler, which are used for cosmetic treatments and are Schedule 4 medications. Despite this, the practice remains widespread partially due to a lack of enforcement and consequences.
“The TGA aims to maximise engagement and encourage voluntary compliance by offering published guidance and targeted educational letters to inform and guide industry on compliance with the Therapeutic Goods Act 1989,” a TGA spokesman said.
It is understood the TGA’s monitoring is ongoing, with more letters to be sent in coming weeks.
Operation Redress is a consumer advocacy group that investigates noncompliance across multiple industries. A lot of the group’s work has focused on surgical and non-surgical aesthetics.
“The letters are a welcome increase in enforcement activity and this move is important in realigning this industry with the rest of the medical sector,” co-director Michael Fraser said.
“However, after much discussion with a range of industry groups, marketing companies and practitioners, most intend to remain somewhat non-compliant for commercial reasons. The recurring sentiment is clear – why comply when nobody is getting fined?”

The group has examined advertising compliance specifically in cosmetics and Mr Fraser can recall finding only two compliant websites out of hundreds.
“This industry needs not only strict regulation, but also strong enforcement,” he said.
“These medical treatments that carry real risks are being marketed as beauty treatments. Social media advertising of Botox and filler is often misleading and downplays risks.
“Many injectors privately tell us about harm they are aware of, with few reporting the conduct to (the Australian Health Practitioner Regulation Agency). This includes reckless behaviour, botching patients, gaslighting patients, use of Non-Disclosure Agreements, and storing medicines in bar fridges.”
Mass audits are also happening in New South Wales where the state’s Pharmaceutical Services Unit is understood to be examining cosmetic clinics and the companies that supply them with Schedule 4 medications.
This comes in the wake of a series of high-profile incidents of concern, mostly in unauthorised clinics, although it is understood that breaches were uncovered at only a handful of businesses.
In one notice, seen by The Australian, the unit says it was concerned about a clinic’s “possession” and “administration of restricted substances” including those used for filler and anti-wrinkle injections.
Legislation governing S4 medications is set at a national level, but each state and territory is responsible for its own interpretation and enforcement, meaning circumstances can vary between jurisdictions.
“Since March, the NSW Health Pharmaceutical Services Unit has conducted more than 100 audits of many types of businesses,” a NSW Health spokeswoman said.
“Of these, four cosmetic clinics were found to have been in breach in some aspects of the Poison and Therapeutics Goods legislation.”
It is believed those clinics are now under active investigation.
Meanwhile, in Queensland, the industry is continuing to come to terms with newly enforced rules.
In December 2024, Queensland Health issued a fact sheet to the industry to help explain existing legislation governing Schedule 4 medications. The legislation has been in effect since 2021 but few in the industry were aware of it.
The fact sheet caused widespread confusion and the department issued newly worded advice in early May that is explicitly clear. However, the advice runs counter to how the majority of clinics operate.
Under the rules, S4 medications can only be held on a premises in Queensland if a doctor or nurse practitioner has exclusive custody of the stock. Registered nurses cannot have custody of the stock, including on consignment, and they cannot order or pay for S4 medications even if they do so under the name of a doctor or nurse practitioner.
For clinics where there is no doctor or nurse practitioner on-site, the medication needs to be dispensed by a pharmacy for each individual patient, and the stock can only be on-site for it to be administered.
Most cosmetic clinics in Australia are nurse-owned, with no doctor or nurse practitioner on-site. Instead, they hold large quantities of medication on consignment under the name of an affiliated doctor or NP. When a patient arrives for an appointment there is a brief consultation via telehealth where the patient will be issued with a prescription and then have the treatment administered, often by a registered nurse.
A routine appointment takes around 15 minutes and it is a model used in individual clinics as well as large national chains. Queensland’s advice makes it clear that model is no longer legal there, and businesses need to find new ways to operate.
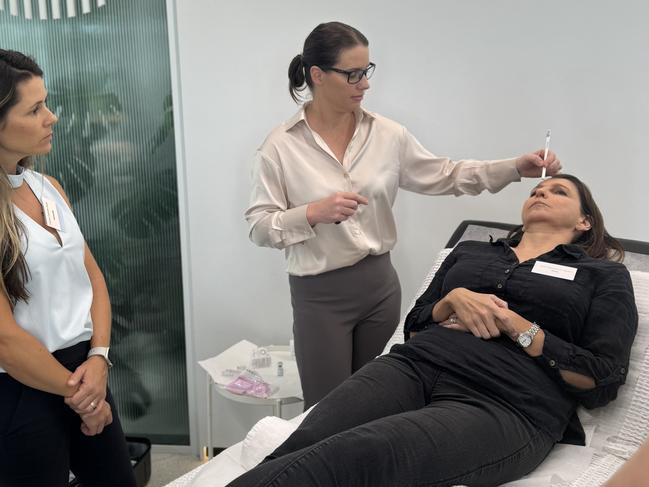
Toni Connor is medical director of Allure Pacific Aesthetics, which provides training, telehealth and S4 medications to its members using what’s known as a pharmacy dispense model.
It provides services to around 450 cosmetic clinics, of which approximately 100 are in Queensland. She said while business models do need to change, the rules are workable.
“What we are now doing is making sure that all S3 and S4 products are labelled prior to being sent out,” Dr Connor said.
“Even doctor-led clinics, unless the doctor is physically in the clinic seeing the patient and all but injecting the patient, that’s the only time the doctor doesn’t have to dispense the product.”
Under the model, a patient attends an initial consultation with a nurse and a doctor is called via telehealth, a treatment plan is created and any prescriptions issued, then the patient leaves. APA then dispenses the medication specific to the patient and it is administered at a later appointment.
“The first consultation is not designed to treat the patient, you shouldn’t be having walk-ins,” Dr Connor said.
“You should be consulting the patient, completing an adequate assessment, and letting them go away and think about it, and then come back for their treatments. Realistically, it’s not about scaling that product, it’s about making sure that you’re being ethical in your practice.”
Practitioners have told The Australian some medical indemnity insurers have advised their members their cover is only valid if they are compliant with the laws and associated fact sheet.
It is understood some companies are looking to test the definition of what “on-site” means.
Dr Connor is deeply concerned junior doctors could now be targeted by various aesthetics groups to deliver prescribing and oversight services for clinics in ways that may not be legally compliant. She fears doctors are not being fully informed of their responsibilities, which could jeopardise not only their insurance but their medical registration. She describes the situation as “dangerous”.
Avant Mutual specialises in medical indemnity insurance for doctors. Georgie Haysom, the group’s general manager of advocacy, education and research, said doctors have a professional obligation to comply with all relevant legislation. Their cover also depends on it.
“Doctors must not remotely buy a stock of cosmetic injectables for a nurse-led cosmetic injectables clinic if they don’t work there and don’t have control over the medication,” she said.
“Doctors also must not prescribe a cosmetic injectable for administration by someone else without first having a consultation with the patient and checking the credentials of the person administering the injectable, because the prescribing doctor is responsible for the management of the patient for whom they prescribe.
“Doctors can, however, prescribe a cosmetic injectable remotely if they consult with the patient first and then the patient takes the medication to the clinic to be administered.”
For a lot of nurses in Queensland, the updated advice has caused anxiety and concern because they have realised they are non-compliant with the legislation and are working to find solutions.

There is also widespread pushback to the laws from nurses who say it will be expensive to comply with the rules. A petition and social media campaign have been launched and lobbyists recruited to pressure politicians to intervene and create caveats. The campaign is particularly targeting Queensland Health Minister Tim Nicholls and Women’s Minister Fiona Simpson.
However, it appears both are holding firm. So too is the regulator, which has rejected a common industry rumour that there is a grace period in place until July. The state regulator told The Australian no such grace period exists, especially given the rules have been in place for four years.
Nurses are also concerned the crackdown is sexist and targets a female-dominant industry, with many questioning the idea that the laws are about safety. It’s an idea rejected by patient safety advocate Maddison Johnstone, another co-director at Operation Redress, who says there is a lack of data around patient harms.

“Queensland and national regulators have also increased compliance efforts in the medical cannabis and vaping industries, which voids the argument that cosmetic injectors are being unfairly targeted because they are female-dominated,” she said.
“There is no mandatory obligation for injectables practitioners to report their complications to a regulator. We again ask industry to join us in calling for a mandatory government register of injectable patient complications, so that this data can be collated.
“What we do know is that patients have experienced permanent vision loss as a result of cosmetic injectables. Those patients have had their autonomy impacted.”
Know more about the story or have a tip off? Contact the reporter: pennytimms@protonmail.com




To join the conversation, please log in. Don't have an account? Register
Join the conversation, you are commenting as Logout