Legal stoush looms over cosmetic injectables rules
Businesses are lawyering up after Queensland Health said only clinics with doctors onsite can buy and store Botox and filler.
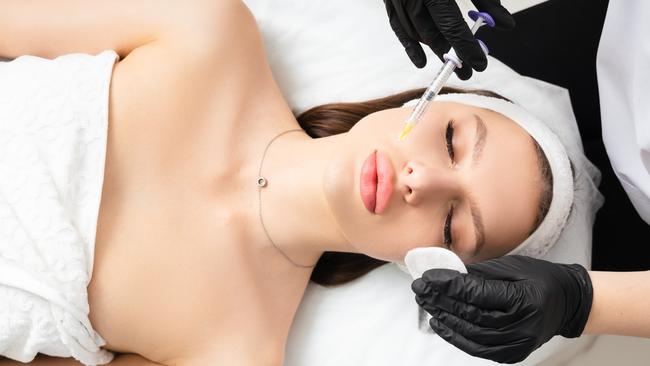
A potential legal stoush is brewing in Queensland where hundreds of cosmetic injectables businesses have been told their operating models don’t comply with regulations.
Multiple operators have exclusively told The Australian they have sought urgent legal advice to decipher the Queensland Health directive, which was published last month but began circulating widely only last week.
On December 9, 2024, Queensland Health published a five-page fact sheet to outline the industry’s “regulatory obligations” under the state’s interpretation of federal legislation around cosmetic injectables. The five-page document mostly focused on substances colloquially known as Botox and filler which are listed as Schedule 4 substances and are available only with a prescription.
When used for cosmetic purposes, the substances are injected into a person’s face to try and reduce the look of fine lines and increase the fullness of cheeks, lips, jaws or under the eyes. Their use has been popularised by Hollywood and reality TV stars including the Kardashians.
While the treatments cost hundreds of dollars, they are often cheaper than other surgical anti-ageing treatments so have become wildly popular and easily accessible.
In Queensland, hundreds of beauty clinics offering cosmetic injectables are owned or operated by nurses – typically registered nurses. They then have partnerships with telehealth companies that provide doctors to prescribe the medications. Those same telehealth companies also offer training and wholesale supply of the drugs.
But according to the advice recently published by Queensland Health, it is unlawful for registered nurses, enrolled nurses, admin staff or other unauthorised persons to buy cosmetic injectables for a beauty treatment or cosmetic business, even “on behalf of” or “with the approval of” a doctor or nurse practitioner. Nurse practitioners are the most highly trained of nurses and sit above RNs.
“This means stock can only be delivered to a place where the authorised buyer, such as a medical practitioner or a nurse practitioner, is physically practising from,” the document says.
“Doctors and nurse practitioners cannot buy stock for a place that they do not practice from, which includes locations for which telehealth is provided.”
It is those two sentences that are causing the mass confusion.
Queensland Health maintains the information is not new. However, multiple people from within the industry dispute that and say it does not align with common practice. It would also mean hundreds of businesses have been operating illegally for years, with no reprimand or enforcement of the law.

The Australian has spoken with multiple business owners who say they have engaged with lawyers to examine their obligations under the Medicines and Poisons Act 2019.
One of those is Juv’ae. It offers telehealth prescribing, training, and wholesale supply of cosmetic injectables to around 500 beauty clinics across the country.
“We have consulted legal representatives to review the fact sheet and ensure compliance,” Juv’ae’s directors Melissa Isaia and Nicole Schmid-Sanele told The Australian.
“Our priority is to safeguard our nurses and provide clarity on any operational adjustments required under the current regulatory environment.
“While we recognise that the fact sheet introduces interpretations, any required changes will be assessed and implemented promptly to ensure compliance while maintaining the highest standards of patient care,” they said.
The directors said the cosmetic injectables industry had experienced rapid growth and acknowledged regulation has a role to play in maintaining safety and standards. However, they said what was also critical was “transparent engagement with stakeholders”.

“Should Queensland Health clarify the interpretations outlined in the fact sheet, Juv’ae will ensure that all necessary adjustments are made to conform to regulatory requirements, ensuring our nurses remain compliant and patient safety is prioritised,” they said.
In a statement to The Australian a spokesperson for Queensland Health said “there has never been a requirement that a doctor must be the administrator or be on site for the administration of cosmetic injectables”.
“Doctors can provide oral or written prescriptions to nurses to administer medicines in accordance with the act,” the spokesperson said.
The department said clinics could operate using a telehealth doctor to prescribe. However, registered nurses “are not able to purchase stock of Schedule 4 medicines”.
“Only a doctor or nurse practitioner can purchase this stock,” the spokesperson said.
While the legislation in question is federal, the Australian Health Practitioner Regulation Agency said it was up to the states and territories to regulate access to the drugs and poisons.
“All practitioners are expected to know and comply with the relevant legislation of the jurisdiction in which they are practising,” an AHPRA spokeswoman said.





To join the conversation, please log in. Don't have an account? Register
Join the conversation, you are commenting as Logout