World wants AstraZeneca Covid-19 vaccine, says CSL
Biotech giant CSL insists that despite fading confidence about the AstraZeneca vaccine rollout, production at its Melbourne facility remains on track.

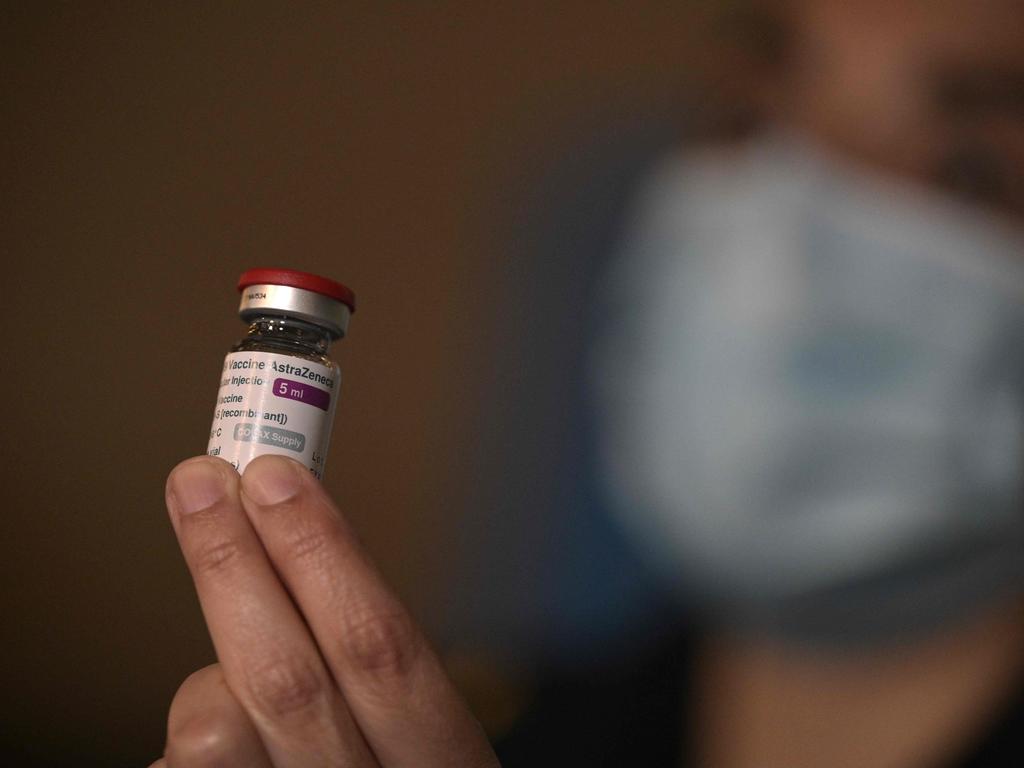
Biotech giant CSL insists that despite fading confidence about the AstraZeneca vaccine rollout, production at its Melbourne facility remains on track, with other countries clamouring for the jab and declaring they will accept it.
Australia’s federal government recently revealed plans to progressively phase out the AstraZeneca vaccine from the nation’s inoculation program, because of a downturn in local demand because it has on rare occasions been linked to blood clots in younger patients.
Despite the setback, CSL’s team of scientists are full steam ahead with production of the vaccine in Melbourne.
During a rare tour of the company’s fast-growing advanced manufacturing facilities, Chris Larkins, senior vice president of operations for the company’s vaccine arm Seqirus, said that AstraZeneca vaccine doses would be lapped up by overseas countries, where Covid was still rampant.
“Since Australia has made the decision to lift the (AstraZeneca) age to 60, which is very much an Australian decision, based on what’s happening in Australia and the lack of any sort of real transmission of Covid, we’ve had governments calling us up from around the world saying ‘we’ll take it’,” Mr Larkins said.
“This product will be manufactured, and it will go to other countries, who probably need it more than we do anyway, and it will go and save lives in those countries.
“We’re talking to our staff around that so there isn’t that feeling of deflation over that change in Australia. We’re considering it a global supply from our end now, to support the global pandemic.”
The executive said that the World Health Organisation would handle the distribution of the surplus vaccines, which were likely to go to lower-income countries.
CSL had a 50 million dose deal with the federal government, an agreement that still stands despite the government changing its advice to recommend Pfizer for people between 16 and 60.
Under a revised rollout program, the government’s latest figures show that additional supplies of Pfizer’s mRNA Covid-19 vaccine will become available locally from September, alongside a new supply of Moderna’s vaccine.

Mr Larkins said CSL had released over 10 million doses of AstraZeneca to the government, and had now produced about two-thirds of its 50 million target.
The federal government has committed to donating 20 million vaccine doses abroad, and has currently provided about 300,000 doses to the Pacific and East Timor.
“Australia will be doing its part, as we already have been, committing some 20 million doses as part of that effort here at the G7 Plus in Cornwall,” Prime Minister Scott Morrison said at the G7 summit last month.
“These 20 million doses will go to support our region to ensure that we continue to exercise our responsibility as part of a broader global responsibility to combat this virus.”
Once the AstraZeneca roll out is complete, CSL will turn its attention to preparing Australia for future pandemics.
“In Melbourne now we’ve got the capacity to go through the whole process, from drug discovery through to the production of material, toxicology studies, and then manufacturing,” CSL’s chief scientist Andrew Nash said. “It is really significant R&D work.”
Key to the company’s lofty ambitions is a new $800m hi-tech facility at Tullamarine in Melbourne, which will produce influenza vaccines using cell-based technology for use in influenza pandemics and seasonal vaccination programs.
Once complete by mid-2026, the company says the plant will be the only cell-based influenza vaccine manufacturing facility in the Southern Hemisphere.
Most current influenza vaccines are egg-based, and require more than 18 months of preparation and many chickens.
CSL is also investing $900m in a plasma facility at Melbourne suburb of Broadmeadows.
This is in addition to $1bn it has already invested in the site.
CSL executive Trisha Stewart said the building would allow the company to process some 9.2 million litres of blood plasma every year.
“We are supporting Australian patients to ensure they have product available into the future, and we are demonstrating what’s possible with Australian manufacturing and Australian labour,” she said.
Mr Larkins also said CSL was in talks with government about a potential mRNA vaccine.
However, she said that it was too early to determine what a potential partnership might look like.
“Those decisions are still playing out,” he said.
“That plant is really looking for the next pandemic, not necessarily the one we’re currently in at this period in time.”





To join the conversation, please log in. Don't have an account? Register
Join the conversation, you are commenting as Logout