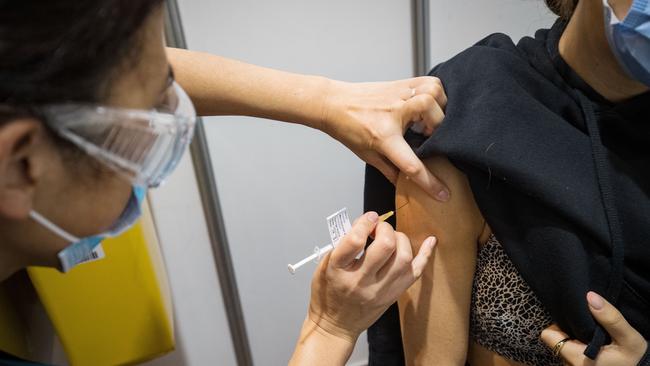
Answering the vaccine call: Australia’s response to Covid-19 is to be hailed, not feared

When Covid-19 hit with full force in March 2020 the medical, scientific and research communities moved quickly; everyone dropped what they had been working on, expertise was pooled and a unified global emergency response was mobilised. This occurred in parallel to the rapid activation of our health systems. Private providers such as Healthscope, alongside the public hospitals, rushed to support the wider government pandemic response.
It has culminated in an unparalleled sharing of resource, knowledge and expertise and in the development of over 200 vaccine and therapeutic candidates to fight the common foe: Covid-19. Three of these vaccines have now been deployed in over 1.3 billion doses worldwide with a compelling impact on the pandemic.
And yet there are those who point to this speed and honing of science with suspicion, as a risk, rather than as an unprecedented achievement. The implication is that the rapidity of our worldwide response has somehow equated to recklessness and the bypassing of adequate scrutiny and regulation.
Nothing could be further from the truth.
Safety and outcomes
The emerging evidence from countries such as Israel that now have the majority of their population vaccinated, or Britain and the US, have shown not only that these vaccines can be administered safely with very few side effects, but that vaccine efficacy is nothing short of stunning.
Hospital admissions and serious illness are down more than 80 per cent in particular, in vulnerable older groups, and the chance of infection in countries where transmission is rife has been reduced to minimal levels. Where before outbreaks in residential aged care were a major risk of the pandemic, with high vaccination rates these outbreaks have decreased by more than 30 per cent and transmission of the virus has been interrupted.
But how has it been possible to produce a safe and effective vaccine in crisis and at pace?
An old foe
Coronavirus is not new. Severe Acute Respiratory Syndrome (SARS) and Middle Eastern Respiratory Syndrome (MERS) are both coronaviruses and were prominent in outbreaks in 2003 and 2012 respectively. A great deal of research was undertaken to understand the structure and behaviour of these viruses and to create vaccine technology to combat them. Thankfully, vaccines for these were not required on scale, but it laid solid foundations for the work on Covid-19.
The technology developed during this time, which used the genetic coding of a virus to develop a vaccine, allows for much quicker production of a vaccine than the older, slower method of capturing a virus, learning how to safely deactivate and then reinject the virus, all while maintaining safety and an adequate immune reaction.
In practice, this meant that the Covid-19 virus had genetic sequencing performed within 10 days of the first atypical pneumonia being identified in Wuhan, China. And within 10 weeks of Chinese scientists publishing this genetic sequence, the first few companies had a vaccine candidate ready to begin trials.
Clinical trials
Never has one virus been studied so intensely by so many. Covid-19 vaccine trials have been run with mass participation in the hundreds of thousands, in multiple locations with rampant background infection rates on a scale never before seen.
Many have assumed that the relatively slow pace of previous vaccine development was based purely in meticulous safety; therefore equating speed to the abandonment of safety.
In fact, one of the key limitations in faster development of vaccines has always been simple economics. Clinical trials are expensive and if the vaccine does not turn out to be a success, any investment in the vaccine is lost. Pharmaceutical companies will run smaller trials, stop and assess the data and the potential market, and then decide if it is worth risking more investment in the technology.

But in the case of the Covid-19 vaccine, this economic risk assessment process was simply invalid. Billions of dollars poured in from all over the world, from governments pre-purchasing orders, from philanthropy and from private enterprise, resulting in the instant funding of more than 200 possible vaccine candidates. This removed the economic risk from pharmaceutical companies and allowed mass trials to be run concurrently across the globe.
The timeline of the Covid-19 vaccine cannot be seen as an indicator of safety, but instead we should look to the unparalleled intensity and scale of the Covid-19 vaccine trials.
Scrutiny and approvals
The threshold for approval of the Covid-19 vaccines in Australia has been no different to that of any other vaccine that many of us have received over a lifetime. The TGA approval in Australia was not an emergency approval, but went through the full regulatory process expected of any other vaccine. With this should come the same level of reassurance that comes from trusting the TGA and its regulatory processes across all medications.
If anything, data and approvals for the Covid-19 vaccine have received more intense and higher-profile scrutiny than any other vaccine in history.
Everything about this pandemic has been unprecedented, including the integrated efforts of scientists, ethicists and regulators who have worked around the clock to develop Covid-19 vaccines. The work done during the last 12 months will change the vaccination landscape forever.
Victoria Atkinson is Healthscope’s chief medical officer and a cardiothoracic surgeon.




When bushfires hit Australia early last year the country moved quickly; everyone dropped what they were doing, expertise was pooled in taskforces and a rapid emergency response was mobilised. No one questioned the capacity or skills of emergency services to keep people safe and to fight the common foe. As a nation we trusted the expertise, training and experience of our experts to execute these skills in crisis and at pace.