CSL sets sights on Covid-19, influenza ‘superjab’ as it puts coronavirus back on its R&D agenda
Combining vaccines for Covid-19 and influenza into a single shot is edging closer to reality thanks to self-amplifying messenger RNA technology, and CSL is getting in on the action.
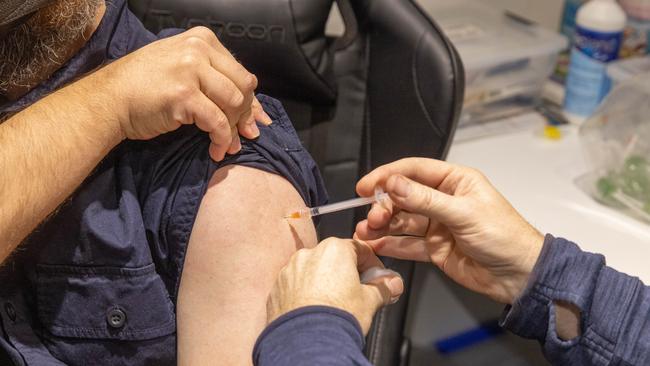
CSL says a superjab – combining Covid-19 and influenza vaccines into a single shot – is in its sights as it reveals its next suite of potential blockbuster drugs and therapies under its $1.2bn research and development program.
Australia’s biggest health company updated investors on its drug development timeline – including when its next big name products are likely to hit the market – at its R&D day on Thursday.
The briefing came a day after CSL secured a licensing deal with Arcturus Therapeutics to access its late-stage amplifying mRNA vaccine platform technology, which includes a Covid-19 vaccine candidate.
After chief executive Paul Perreault said previously that creating a superjab for Covid-19 and influenza would be a “big ask”, the company now says the rapid development of self-amplifying mRNA technology makes such a shot possible.
Asked if the deal with Arcturus aimed to bring the goal of delivering a combination Covid-19 and influenza vaccine closer to reality, CSL’s vice-president overseeing the clinal development of vaccines, Jon Edelman, said such a move made sense.
“We’re looking at that and kind of take them one at a time,” Dr Edelman said.
“One of the advantages of the self-amplifying technology and the ability to use smaller doses for any one vaccine means that a combination vaccine might be something that will be better tolerated because the total amount of the vaccine that you need is is less than you’ll see with the more conventional RNA vaccines.”
CSL has been developing what it calls the next generation of flu vaccines, which use mRNA technology – the same used in Pfizer and Moderna’s Covid-19 jabs.
Andrew Nash, CSL’s chief scientific officer, said Covid-19 would likely emerge as a seasonal virus, similar to influenza, in which vaccines could be matched to strains identified by the World Health Organisation.
“That hasn’t happened yet but our sense is there will be at some stage seasonal Covid vaccinations as well as seasonal influenza vaccination, so it makes sense to be looking at those things and having the technology that works well both in the context of individual virus but also in terms of the combination of virus and multiple antigens from within the one virus as well.”

Messenger RNA vaccines have been in development for many years but it has taken the Covid-19 pandemic to catapult it into the mainstream. It works by sending instructions to the body’s cells to make a particular type of protein that stimulates an immune response to protect against infection.
This is unlike the traditional method of manufacturing flu shots, which uses chicken eggs to grow the virus, before weakening it or killing it to develop a vaccine.
But Dr Nash said the technology has its limitations and the ability to modify mRNA vaccines to different strands within the one virus season would be “unrealistic”.
“I think the ability to rapidly switch is probably somewhat overstated due to logistics and the timeframe would be highly unlikely to enable that,” he said.
It comes after CSL quietly pulled the plug on developing Covid-19 antiviral treatments earlier this year as it focused its attention on other projects in its $1.2bn drug development program.
The company had been involved in an exhausting fight against coronavirus that began in early 2020, when CSL partnered with the University of Queensland to develop a potential vaccine. The company later joined a global alliance to work on a hyperimmune therapy to combat Covid-19, winning praise from the then US president, Donald Trump.
But both projects failed. The UQ vaccine – despite proving effective – was aborted in late 2020 after it triggered false positives for HIV, and CSL withdrew the potential hyperimmune therapy five months later after it “did not meet its endpoints”.
The refocusing on other projects is about to pay off. The US Food and Drug Administration has accepted CSL’s gene therapy – called etranacogene dezaparvovec – for adults with haemophilia B for priority review.
“If approved, etranacogene dezaparvovec would be the first-ever gene therapy treatment
option for the haemophilia B community. The regulatory filings are supported by results from the pivotal HOPE-B trial, the largest gene therapy trial in haemophilia B to date,” the company said.

It also achieved favourable phase 3 clinical trial results for garadacimab – a treatment for people with hereditary angioedema, a condition which causes swelling under the skin.
“CSL aims to begin filing for approval with global regulatory authorities next calendar year,” the company said.
Meanwhile, its $US11.7bn ($18.4bn) acquisition of Swiss renal treatments company Vifor – now rebadged CSL Vifor – has included a portfolio of therapies in nephrology, dialysis and iron deficiency, including late stage drug development.
Dr Nash said CSL’s broader R&D program would also serve the needs of Vifor.
“Their R&D portfolio isn’t as large scale as what CSL does. We’ll be looking to work very closely with our people, colleagues, to really find the best operating model for R7D going forward,” he said.
“We bought Seqirus (CSL’s vaccine division) back into CSL, it’s CSL Seqirus, and we have CSL Vifor, and we’ll have an R&D organisation that supports each of those organisations.”
The company is also progressing with its biotech incubator in Melbourne, which will house 40 start-ups, after securing $95m from the Victorian government.
“Incubator residents will be working in an innovation-driven environment alongside a large and focused CSL R&D team, enabling opportunities for peer-collaboration, learning and sharing of ideas,” Dr Nash said.
Bill Mezzanotte, CSL’s head of R&D and chief medical officer said: “Our enhanced capabilities across all of our scientific platforms and therapeutic focus areas will help us in our relentless pursuit to deliver on our promise to help patients lead full lives, protect public health and sustainably grow our business in the decades ahead, while providing promising futures for our employees.
“CSL is on the leading-edge of innovation in areas we know well and we have strategically and methodically built a pipeline that has never been more robust with diverse sources of innovation, from in-house and external sources, that include the disruptive scientific platforms of gene therapy and sa-mRNA.”






To join the conversation, please log in. Don't have an account? Register
Join the conversation, you are commenting as Logout