CSL ends Covid-19 research battle, haemophilia B a key element of $1bn R&D program
CSL is turning to other projects in its $1bn-a-year research program, ending its aim to develop antiviral Covid-19 treatments.

CSL, the nation’s largest pharmaceutical company, has quietly pulled the plug on developing Covid-19 antiviral treatments as it steams ahead on other projects in its $1bn-a-year research program.
The move marks the end of an exhaustive fight against the coronavirus that began in early 2020, when CSL partnered with the University of Queensland to develop a potential vaccine. The company later joined a global alliance to work on a hyperimmune therapy to combat Covid-19, winning praise from the then US president, Donald Trump.
But both projects failed. The UQ vaccine – despite proving effective – was aborted in late 2020 after it triggered false positives for HIV, and CSL withdrew the potential hyperimmune therapy five months later after it “did not meet its endpoints”.
The difficulty in CSL – which has some of the world’s top researchers and scientists – developing successful treatment for the virus underscores the broader struggle in taming Covid-19, which has crippled the globe for the past three years.
It comes as the nation battles record infections from the highly infectious but less severe Omicron variant of the virus, with the US and EU issuing warnings about travelling to and from Australia. The only product to gain full regulatory approval was the AstraZeneca vaccine that CSL manufactured under licence in Melbourne – the only factory in Australia producing an approved Covid-19 vaccine.

The company is manufacturing more than 50 million doses of the AstraZeneca vaccine, now known as Vaxzevria – which was initially expected to immunise most people in Australia.
While millions did receive the CSL-produced AstraZeneca vaccine, many others did not after it was linked to rare blood clots, prompting Australian Technical Advisory Group on Immunisation to recommend rival Pfizer for those under 50.
A CSL spokesman confirmed the company was no longer investigating Covid-19 antiviral therapies.
“CSL is proud of the role we have played in bringing the Vaxzevria vaccine to millions of Australians, as well as neighbouring countries. At present we are not investigating any antiviral treatments for Covid-19,” he said.
The company’s hyperimmune therapy for Covid-19 involved using antibodies found in plasma donated from people who have recovered from the virus to help others fight off the coronavirus.
At a White House roundtable attended by CSL chief executive Paul Perreault in July 2020, Mr Trump said the plasma-based therapy was “a more delicate way of doing things”. He has previously advocated the use of hydroxychloroquine to ward off Covid-19, despite his own health officials saying it was ineffective.
“These therapies transfuse powerful antibodies from the blood of recovered patients to help fight those battling the current infection that we all know so well,” Mr Trump said.
But by April last year, CSL chief medical officer and research and development head Bill Mezzanotte said the treatment “did not meet its endpoints to show efficacy in adults”.
“While the results of this particular clinical trial are disappointing, we are proud that as an industry we proactively and collaboratively pursued this work, and that the program may contribute to a growing understanding of this challenging virus and strategies for patient care,” Dr Mezzanotte said at the time.
“Since we embarked on this development program, and throughout the pandemic, we have learned much from our scientific research. Importantly, we learned that as an industry we have the fortitude and capability to quickly work together for the greater good of human health.”
The company is also considering developing its next-generation mRNA vaccine – the technology behind Pfizer and Moderna’s jabs – at its existing facilities overseas rather than at a new centre in Melbourne.

The company planned to build a dedicated mRNA factory in Melbourne, but CSL chairman Brian McNamee said in a statement last month that the Australian government had advised that “it will not advance the company’s proposal to construct an onshore mRNA vaccine development and manufacturing facility”. Instead, the government awarded the contract to Moderna.
Dr McNamee said while CSL had more than $2bn in “expansion projects” under way in Australia, its vaccine business Seqirus had substantial capacity at its overseas centres.
“We are firmly committed to advancing our next-generation sa-mRNA vaccine technology which aims to address some of the challenges presented by the current technology. We will utilise our global network, including research facilities in Cambridge, Massachusetts and clinical scale manufacturing facilities in Holly Springs, North Carolina, to achieve this,” Dr McNamee said shortly before Christmas.
“Seqirus’s research development program will also benefit from the recent multi-year contract with the US Department of Health & Human Services to investigate influenza vaccine technologies including sa-mRNA. This builds on our longstanding public-private partnership to provide a rapid response in the event of an influenza pandemic.
“The company will continue to consider options for an industrial-scale mRNA vaccine manufacturing facility and determine where it is most compatible within our global network.”
While Australia will not host its mRNA development, it is building an $800m cell-based influenza vaccine factory in Melbourne that is scheduled to open in 2026. Cell-based flu vaccines do not require eggs to manufacture, enabling higher quantities to be made at shorter notice – a must in a pandemic.
The Tullamarine facility will replicate Seqirus’s influenza factory at Holly Springs, North Carolina, which has been manufacturing and stockpiling AUDENZ, the first adjuvanted, cell-based influenza vaccine designed to protect against H5N1, known as bird flu.
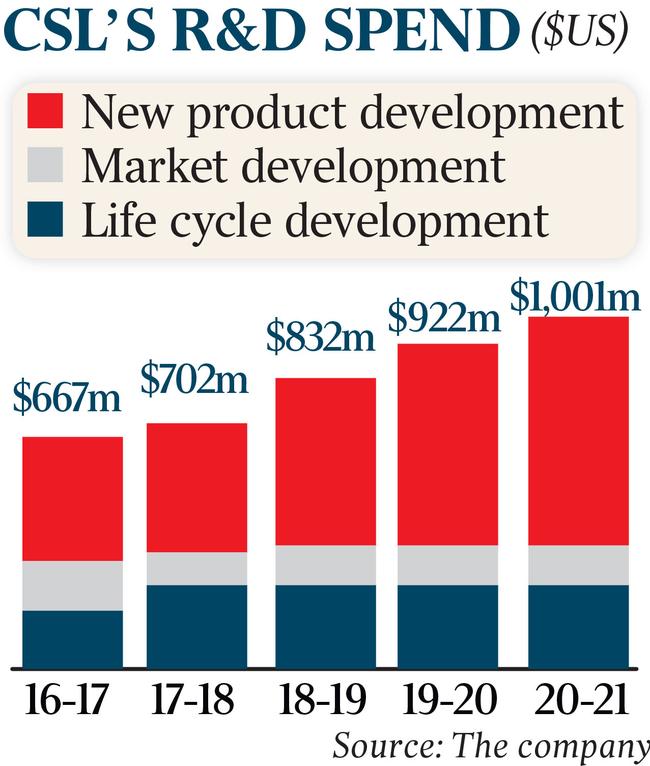
Elsewhere across CSL’s $1bn-a-year research portfolio is a potential genetic therapy to cure bleeding disorder haemophilia B, which Goldman Sachs analysts say could be worth up to $6.70 of CSL’s $258.35 share price.
CSL has partnered with gene therapy company UniQure for its haemophilia B treatment that is now approaching regulatory approval after it achieved success in phase-three trials.
“We now look forward to collaborating with CSL Behring on completing the regulatory submissions that we hope will advance etranacogene dezaparvovec one step closer to reaching haemophilia B patients around the world,” UniQure’s Ricardo Dolmetsch said.
The treatment has been granted breakthrough therapy designation by the US Food and Drug Administration and access to a priority regulatory pathway by the European Medicines Agency.







To join the conversation, please log in. Don't have an account? Register
Join the conversation, you are commenting as Logout