CSL ‘can make 100m coronavirus vaccine doses’
CSL says it can produce 100 million COVID-19 vaccine doses by the end of 2021, if trials involving partners are successful.
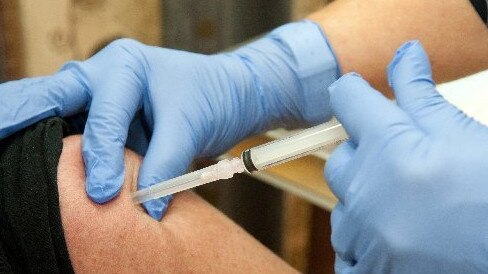
Australia’s biggest health company, CSL, says it can produce up to 100 million doses of a COVID-19 vaccine by the end of next year if clinical trials in Australia and in high-infection areas overseas are successful.
CSL has struck a partnership with the Oslo-based Coalition for Epidemic Preparedness Innovations (CEPI) and University of Queensland to fast-track the development of a COVID-19 vaccine, catapulting it to the forefront of the global battle to fight the coronavirus.
But CSL chief executive Paul Perrault said that while the company and UQ had won international endorsement, it was not a race against other vaccines in development.
“This is a race against the virus and there will be multiple vaccines to supply the globe,” Mr Perrault said.
“We don’t know enough about the virus today to know how long it will be around, if there will have to be additional doses of vaccines in the future, and so it is imperative that everybody work as hard as they can collaboratively.” The agreement formalises CSL’s commitment to lend all its resources to UQ’s vaccine research effort, and comes 25 years after CSL and UQ partnered to develop the cervical cancer vaccine Gardasil.
Meanwhile, CEPI has provided UQ up to $US10.6m ($15.16m) to develop its “molecular clamp” vaccine platform, a transformative technology patented by UniQuest, UQ’s technology transfer company, that enables rapid vaccine design and production.
Already early preclinical results in mice have shown their COVID-19 vaccine candidate produces high levels of antibodies that can neutralise the virus, which has killed 383,000 people worldwide.
UQ is now on track to launch a phase 1 clinical trial next month, which will involve 120 patients, and be funded by CSL and CEPI. While initial results are promising, Mr Perrault cautioned that there was no guarantee of success.
“Research is risky, even at the best of times,” he said.
“While initial results from UQ’s research are very promising there remains a number of critical milestones ahead before we can declare victory with an effective and safe vaccine to protect people from COVID-19.”
The phase 1 trial will test the safety of the vaccine and also help determine the correct dosage. If that is successful, the vaccine will proceed to phase 2 trials, involving 800-1000 people.
UQ professor Paul Young said that unlike the phase 1 trial, which would be conducted in Brisbane, the phase 2 trial would be performed overseas.
“Fortunately right now in Australia the infection rate is pretty low, which is great. But to do the phase 2 efficacy study we need to do it in parts of the world or a part of the world where there are infections taking place,” Professor Young said.
“We are looking very hard with the clinical team now at which countries or cities the studies be done. It will have to be done where there are both infections but also where there is good health infrastructure and where we can gather high-quality data.”
CSL chief scientific officer Andrew Cuthbertson said the company hadn’t budgeted the full cost of the vaccine development.
UQ vice-chancellor Peter Hoj said the vaccine development was at a “critical juncture”.
CEPI chair Jane Halton said multiple vaccine research efforts were needed to defeat the virus.







To join the conversation, please log in. Don't have an account? Register
Join the conversation, you are commenting as Logout