Coronavirus treatment: Antimalarial drugs approved by FDA to fight COVID-19
Two antimalarial drugs that have shown promising results in small anecdotal studies have been granted emergency approval for millions of doses across the US.
Two unproven drugs have been approved for widespread use across the US to treat coronavirus.
The Food and Drug Administration (FDA) has fast-tracked emergency approval of the drugs, which haven’t yet been proven to cure or assist in the treatment of coronavirus, according to The Washington Post.
The federal agency, which is responsible for protecting and promoting public health through the control of drugs and other products in the US, said it was worth pushing the drugs into hospitals if they could help save lives.
"It is reasonable to believe that chloroquine phosphate and hydroxychloroquine sulfate may be effective in treating COVID-19,” FDA chief scientist Denise Hinton wrote when granting the emergency approval.
RELATED: Follow the latest coronavirus updates
RELATED: Follow the latest world live coronavirus updates
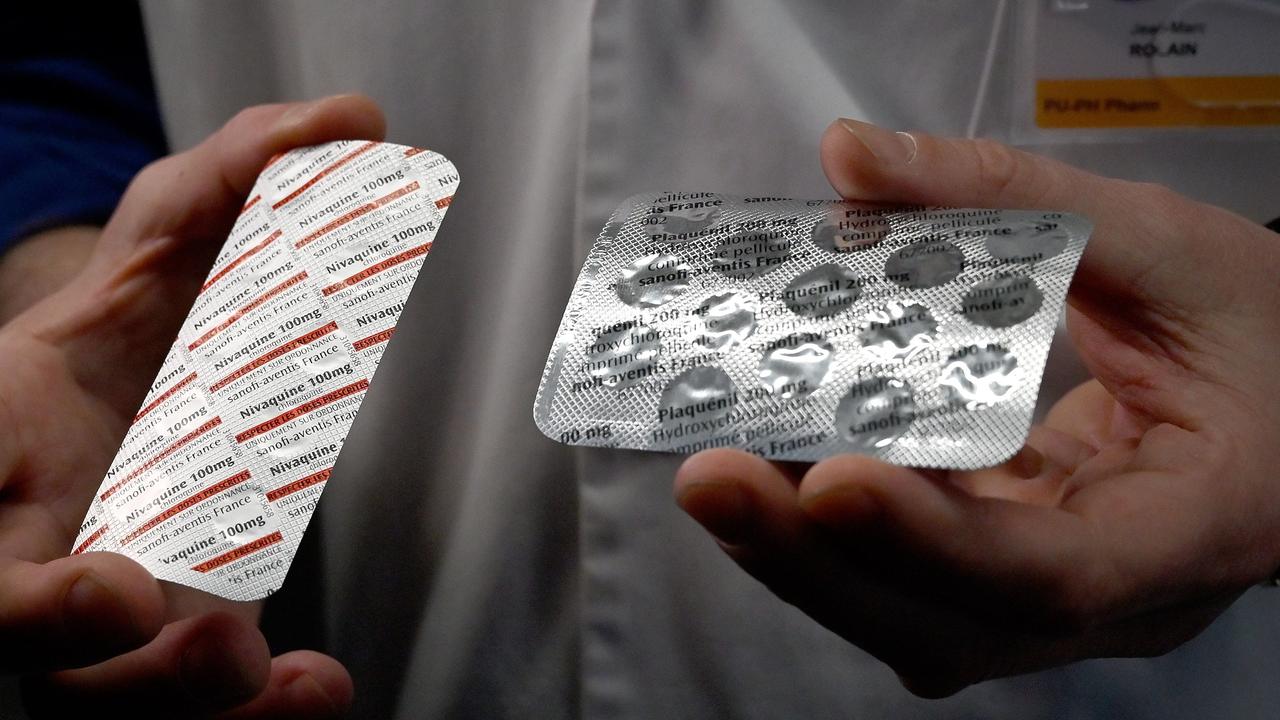
The antimalarial drugs hydroxychloroquine and chloroquine have shown in small, anecdotal studies to relieve the acute respiratory symptoms of patients with COVID-19. According to the report, the drugs show a possible benefit and the ability to clear an infected patient’s symptoms.
Despite the promising early results, the drugs, which have both been in the health system for years, have well-known side effects that health experts have warned could become a problem with wider use. These include affecting patients who have heart conditions, causing possibly fatal side effects to the heart rhythm in patients also using antidepressants.
The usual recommendation is to undergo wide screening to prevent the possibility of death from treatment.
Doctors have warned that approving millions of doses could lead to drug-induced deaths.
The drug is used for a short period of a few days to fight the virus, but doctors also warn that longer term use of the drug can cause vision loss.
The FDA chief scientist said the approval body deemed it an effective treatment.
RELATED: All your questions about coronavirus answered
RELATED: Blood types more at risk of infection

In the US, which now has the most cases of the coronavirus in the world at more than 153,000, doctors have reportedly been “off label” prescribing the antimalarial drugs to their patients for some weeks. The drugs have also been publicly endorsed by US President Donald Trump as a promising treatment.
Pharmaceutical companies Novartis and Bayer will provide millions of doses of the drug to the US Government to distribute via the country’s Strategic National Stockpile.
The drugs were requested by the Biomedical Advanced Research and Development Authority, a US office that works against bioterrorism and pandemics.
Hydroxychloroquine, which has recently experienced a surge in demand, is mostly used by patients with lupus and rheumatoid arthritis.
Many of those patients have since been unable to fill their prescriptions.
The drugs have also been endorsed in Australia by billionaire businessman and former federal politician Clive Palmer, who personally splashed out $1 million of his own money to buy doses of the drug.
They’ll be trialled in 50 hospitals throughout Queensland, according to Mr Palmer.