Australian women cite debilitating pain from contraceptive Essure, lose class action against manufacturer Bayer Australia
The Supreme Court has rejected claims of thousands of Australian women who said a metal coil contraceptive device left them in debilitating pain, saying their symptoms could not be linked to the birth control.

Police & Courts
Don't miss out on the headlines from Police & Courts. Followed categories will be added to My News.
More than 1000 Australian women who suffered chronic pain have lost a class action lawsuit against a pharmaceutical giant after a judge rejecting their claims that a contraceptive device was responsible for their health woes.
Victorian mother Patrice Turner led the legal action against Bayer Australia, the manufacturer of Essure, a metal coil contraceptive device which she and 1400 other women claimed caused problems such as extreme pain and inflammation.
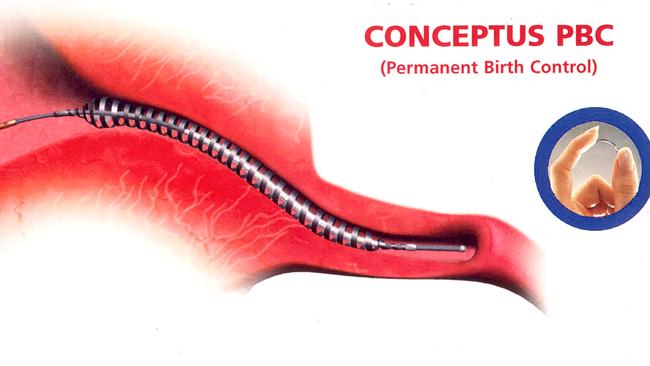
During a 12-week trial in the Supreme Court of Victoria, evidence was presented of suffering young women requiring hysterectomies and other invasive surgeries to remove the devices.
The class action, commenced by Slater and Gordon, alleged Bayer Australia and its distributors breached consumer protection laws and their duty of care to consumers in producing a device that has serious risks of harm of this nature.
It also claimed Bayer failed to warn consumers about safety risks so that women could make an informed choice about their contraceptive options.

Justice Andrew Keogh on Tuesday found that all claims against the pharmaceutical company had failed and that the device was not at fault for the womens’ ill health.
The decision was announced in front of a packed courtroom and hundreds of online observers, with lawyers for Ms Turner already discussing potential avenues for appeal.
“Chronic pelvic pain and abnormal uterine bleeding commonly affect women of reproductive age,” Justice Keogh said.
“There is a broad range of potential causes of both disorders.
“The clinical evidence does not support general causation. I am not satisfied Essure can cause ongoing chronic inflammation in some women, resulting in chronic pelvic pain, dysmenorrhoea or abnormal uterine bleeding,” Justice Keogh said.

His Honour also did not accept the claim that Essure could break, fragment or fatigue once implanted in the body and said adequate warnings about the product were issued.
Essure was inserted into the bodies of thousands of Australian women from about 2000 until it was withdrawn from sale by Bayer in mid-2018.
According to Slater and Gordon, it had been linked with a range of serious conditions and complications from chronic pain and bleeding to perforation of the fallopian tubes and other organs.
The trial heard evidence from expert witnesses involved in gynaecology, epidemiology, pathology, and biomaterials.
Bayer said the decision to withdraw Essure from Australian and overseas markets was not due to safety or quality reasons but due to reduced patient interest in permanent birth control.
In 2020, Bayer paid $1.6 billion to settle a US class action against Essure due to similar claims of serious health complications.
A Bayer spokesperson said: “Women who currently have Essure may continue to confidently rely on the device. If a woman with Essure has questions or concerns about the device, then we encourage her to speak to her healthcare professional.”




