Fundraising decides the fate of kids like Marko, as family prays for cancer breakthrough
There is nothing Jelena and Andrija Magic wouldn’t do for the chance to save their two-year-old son, Marko, who has a rare form of cancer, and the Melbourne couple is now pinning their hopes on an experimental treatment only available in the US. But it doesn’t come cheap.
VIC News
Don't miss out on the headlines from VIC News. Followed categories will be added to My News.
At the hospital bedside of her sleeping young son, Jelena Magic’s detective work begins.
In every spare minute she searches online. Late at night she connects with other oncology parents also sitting bedside, but at hospitals on the other side of the world. Early in the morning she trawls medical journals for clues on where to turn next.
There is nothing she wouldn’t do for the chance to save two-year-old son Marko, who has a nerve tissue tumour. But the Greenvale mother is finding that the price of life — even just the chance to try a promising option — is increasingly out of reach.
CANCER PATIENTS FUTURE-PROOFING FERTILITY
OUR HEALTH EXPERTS HEALING KIDS AROUND THE WORLD
PRECISION MEDICINE A REALITY KIDS WITH CANCER
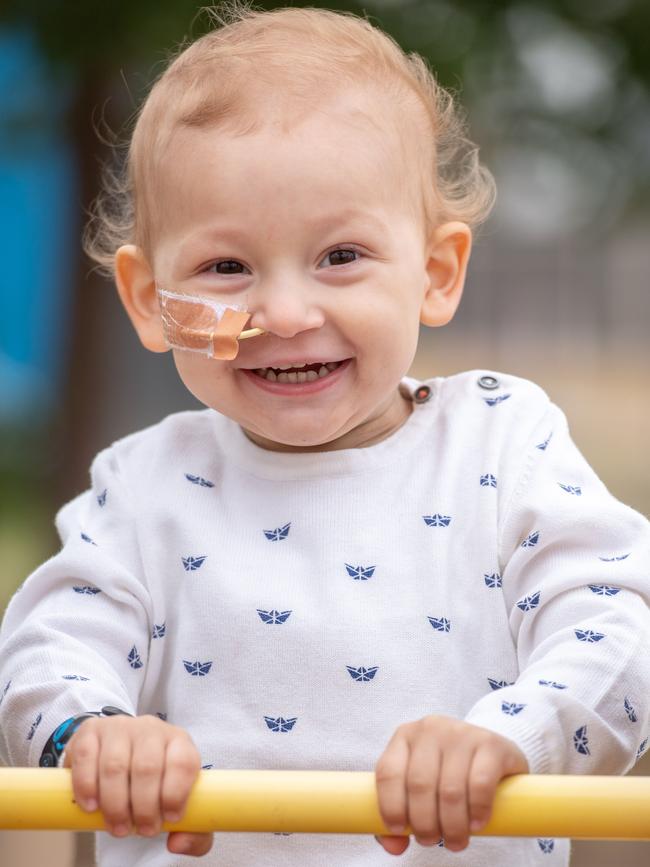
The type of neuroblastoma that Marko is being treated for in Melbourne has a very high chance of returning. The odds for survival drop to around 10 per cent if it does come back.
But with nothing left in Australia but to follow the same aggressive quinella of treatments if Marko relapses, Mrs Magic and husband Andrija are now pinning their hopes on an experimental treatment for neuroblastoma that has been developed in the laboratory at the Memorial Sloan Kettering Cancer Centre in New York City.
Cancer cells are lethally sneaky; they fly under the radar of the immune system like a fighter jet. Ten years ago, the American researchers started testing a vaccine that aims to trigger the patient’s immune system to recognise the cancer cells as foreign, so the body’s own defences can mount an attack.
A phase I study, the first time a drug is tested in patients to assess its safety and an appropriate dose, was tested in children who had relapsed for the second time.
“It is having amazing results,” said Mrs Magic.
“About 90 per cent of the kids are still alive at three years, which is unprecedented.”
The larger phase II study, now under way, will test whether the treatment actually works in those who are entering remission for the first time.
They will continue enrolling up to 260 patients over the next year. But it isn’t cheap.
Families outside the US will need about $350,000 to access the year-long trial to cover medical expenses, travel to New York and living costs.

The federal government does pay for the cost of medical treatment overseas and travel expenses through the Medical Treatment Overseas Program.
The intended treatment for a life-threatening condition must be for a “standard form” of treatment not available in Australia, and have a “realistic prospect of a cure and significant extension of life expectancy”.
An average of 10 Australians a year for the past five years have had their overseas treatment paid for in this way, mostly to the US for the highly specialised cancer treatment proton beam radiotherapy.
But with international trials — particularly for rare diseases — gathering pace, and the online world making it easier for patients to connect and see which treatments are working around the world, Australian families and cancer charities want the government to expand its support.
“We are the fifth family we know of with high-risk neuroblastoma who have needed to resort to public fundraising to access options overseas,” Mrs Magic said.
“So much funding has gone into breast cancer, prostate cancer and leukaemia, but because of the low number of children with these forgotten cancers, all available treatment is still part of clinical trials.”
Founder and chairman of Rare Cancers Australia, Richard Vines, said given there was an increasing number of potential treatments being developed overseas — some proving successful in early trials — it was time for a rethink on how patients with no or limited treatment options in Australia could access them.

“There are mainstream major hospitals around the world running trials,” Mr Vines said. “They’re not flaky, nasty clinics.
“There are going to be a lot of new technologies coming through. Clearly the US and Europe are going to be ahead of us, and we need to acknowledge that.
“In some respects it’s a slightly long bow to call them experimental. Particularly for rare cancers, there are clinicians out there who say clinical trials are the only way patients can access the latest and best, because it’s so hard to get them into the everyday health system. It will be an ongoing problem, and it’s not beyond us to fix it.”
Mr Vines said the government should consider funding at least the travel and accommodation costs. “You could have a respected group of clinicians as a review committee, but we could be more flexible,” he said.
A spokesman for federal Health Minister Greg Hunt did not comment on calls to relax the criteria to allow subsided access for clinical trials, but said the Medical Research Future Fund had increased access to national and international clinical trials, and the development of new treatments and cures.
“To date, our government has announced over $36 million under the MRFF rare cancers and rare diseases and unmet needs clinical trials program, supporting 25 first-time clinical trials,” he said.
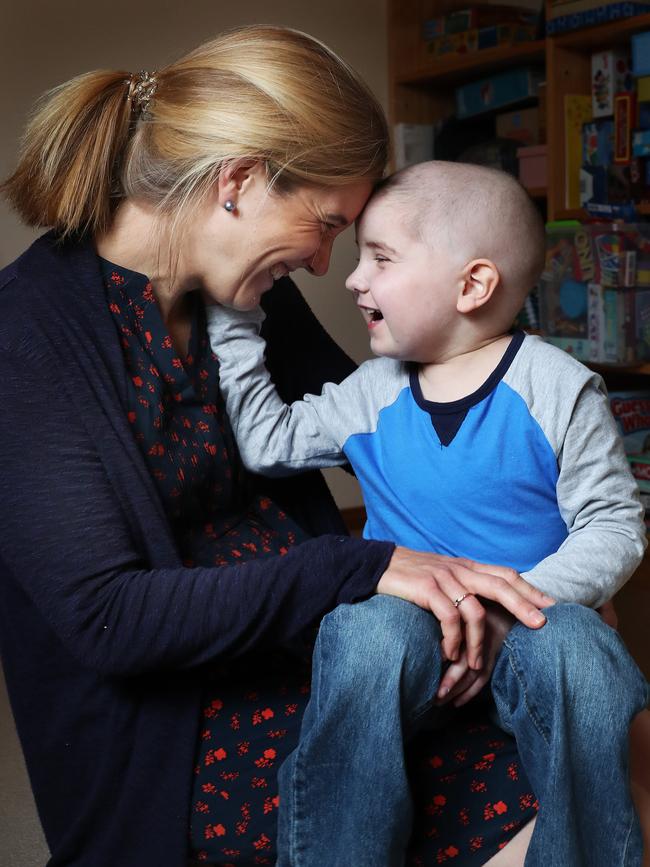
JO Shirran vividly remembers the second job her husband Mark, an emergency physician, took on as the chief medical officer in Operation Chasing Charlie’s Cure. Their son was nine months old when diagnosed with high-risk neuroblastoma, just like Marko.
Dr Shirran researched meticulously and spoke to clinicians around the world to find out about the New York vaccine trial.
“He was relentless. From the minute we got the diagnosis, that day, he researched to find out whether Australia was even the best place for him to be treated,” Mrs Shirran said.
“He didn’t stop until he got the answers he needed. Morning, noon and night he was working on this.
“We were lucky we have that capacity of understanding the medical system. We often speak about families who don’t have a medical background and having to navigate everything is really scary.”
A community fundraising campaign saw the Gold Coast family launch a massive fundraising drive to find the required $250,000.
“It’s a tricky one because I imagine there are a lot of other experimental trials that may not be quite so solid. But I do think it should be based on a case-by-case situation,” Mrs Shirran said.
“Ethically, I struggle with the fact that it comes down to money whether you can save your kid’s life or not.”
The three-year-old’s latest scans this week show he remains cancer free, after finishing the year-long course of seven doses last June.
“He’s suffered considerably to get to this stage. He has hearing loss, infertile, will probably have number of developmental issues as he grows up — it’s inevitable with what he’s had done to him.
“But we are one of the lucky ones and we take each month as it comes at the moment.”
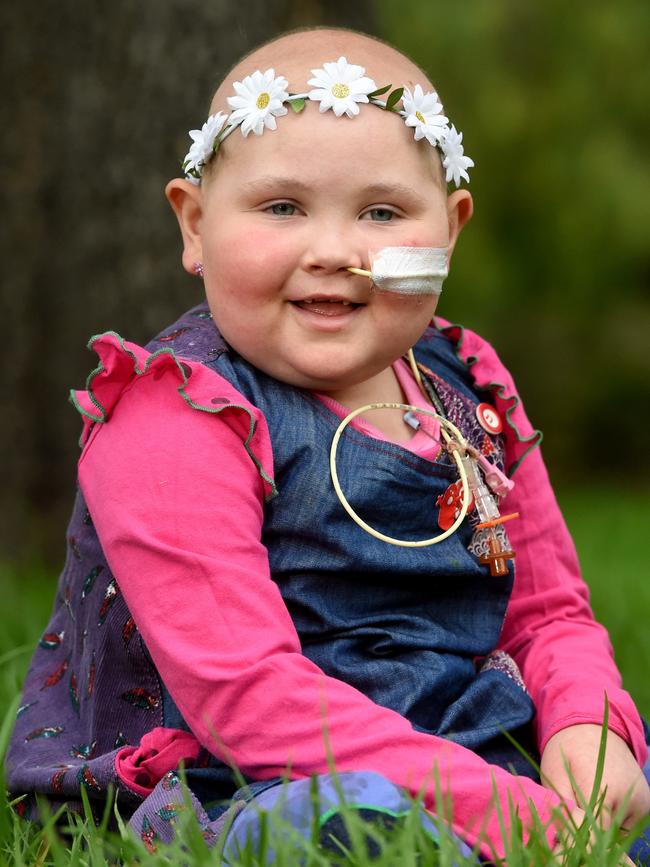
FUNDRAISING campaigns have been launched by many Australian leukaemia patients to access CAR T-cell therapy. The groundbreaking treatment re-engineers the patient’s immune cells so they recognise the cancer cells as foreign and launch an attack.
One of the campaigns featured in the Herald Sun was that of Ivy Steel, who was diagnosed with an aggressive and rare type of acute lymphoblastic leukaemia when she was 2½.
In 2016, as the then six-year-old from Hamilton started fighting cancer for the third time, her family launched an appeal through the Herald Sun and raised more than $630,000.
After relapsing late last year, Ivy and her mum Jenna arrived in the US in early December for treatment at Seattle Children’s Hospital — just as the federal government announced CAR T-cell was approved for use in Australia.
It is still unclear, however, when patients will be able to access this treatment locally, while a plan for how to pay for the expensive therapy is sorted out in Canberra.
On Wednesday this week, Ivy received this long-awaited treatment — the two doses of 13.5 million re-engineered immune cells syringed in an IV infusion. Now they wait.

The Hobart family of six-year-old Ned Isham launched a fundraising appeal on Good Fridaylast year in the Herald Sun, as they faced the prospect of having to find up to $700,000 to access the CAR-T cell.
A community fundraising drive and an anonymous donor, who stepped in at the 11th hour, raised the money need for the family of six to travel to Seattle in August.
Ned’s cancer was returning so rapidly that they could not afford to wait the four to six weeks for their claim to access federal funding to be assessed.
“There needs to be a much quicker turnaround,” Ned’s mum Dr Emily Isham said. “A child’s life depends on having a certain amount of bone marrow disease before they can get treatment. These decisions need to be made quickly. Ned wouldn’t have survived to wait for their funding decision.”
Dr Isham said that while Ned accessed CAR T-cell therapy as part of a trial, they would have qualified for government financial support because of the robust evidence around its effectiveness. “That proof is important because there is a lot of rubbish out there,” she said.
It took a bone-marrow transplant from his sister, the CAR-T cells, and another cell transplant from a stranger to see Ned now cancer free.
JELENA Magic, too, is looking forward to the day where she can say she has tried everything for her boy. “I just want to give him the option of growing up and leading a productive life, just like I wish for my other two boys,” she said.
“We appreciate it’s very expensive to bring these treatments to Australia and initiate clinical trials. The population here will never stack up. But because of those limitations, we need to work better with other hospitals.
“To a parent, it doesn’t matter if the treatment is here or overseas. Every child should get a chance at survival.”
To donate: https://www.gofundme.com/markosmile


