Promising new Alzheimer’s drug Aducanumab awaits TGA approval
A new drug for Alzheimer’s disease — which may halt the deterioration of the brain — is being considered in Australia after accelerated approval in the United States.
NSW
Don't miss out on the headlines from NSW. Followed categories will be added to My News.
It is the ray of hope the 300,000 Australians a year diagnosed with Alzheimer’s have been praying for — a kind of immunisation that may halt the deterioration of the brain.
This “immunisation”, which seeks out and destroys the beta-amyloid proteins that form plaques in the brain and destroy your neurons, has been given accelerated approval for use in the USA.
And submissions for the approval of the drug called Aducanumab are before the Therapeutics Goods Administration (TGA) in Australia.
It has been four decades since Australian Professor Colin Master and his colleagues identified the protein found in the brains of Alzheimer’s sufferers that is thought to cause the devastating decline in memory.
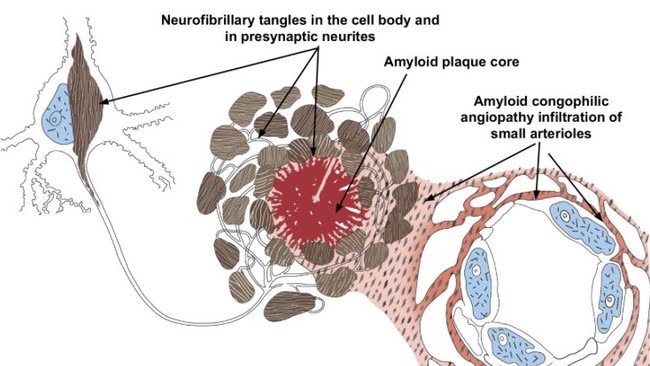
The drug is an antibody engineered to stick to the amyloid molecule that forms plaques in the brains of people with Alzheimer’s. It is thought these plaques form and then damage the brain cells.
“The drug is a biologic and antibody you inject into your blood,” he said.

“This is the first approved drug that clears the amyloid out of the brain. It is fantastic.”
Once the plaque has been removed by the immune system, it is proposed the brain cells will stop dying, preventing future deterioration.
For Port Macquarie mother of two Nell Hawe, who was diagnosed in 2019 with early onset Alzheimer’s at age 50, it means the world.
“It’s very exciting, it’s the first time we’ve had hope, I’d love to get my hands on this drug,” she said.
“I’d really like to see my daughter and son’s future grandchildren, as well as my five grandchildren on my husband’s side, and not just be here in body, be here to remember them and see them.”
For Mrs Hawe, the signs and symptoms first appeared in 2016. As a trainer for dementia carers she recognised them.
“I had symptoms of dementia and they kept putting it down to stress,” she said.
“But on the 21st of April 2016, I found a note on my phone where I had written my symptoms, which were writing lots of notes to remember things, setting reminders on my phone, using people to remind me where I put things.


“My grandfather, my two uncles have passed away and an aunt, all on my father’s side,” she said of the disease.
The accelerated approval in the USA is off the back of two studies, in which some Australians participated in.

The other study was unclear, so part of the US approval of Aducanumab requires Biogen to conduct additional studies.
“The future is even brighter because it is expected the drug will perform much better if you can get it in before you have any serious cognitive impairment,” Prof Masters said.
“Giving the drug to people before the onset of disease, those studies are now underway.”
But it won’t come cheap. The drug will need to be delivered intravenously every four months for a lifetime and Biogen estimates Aducanumab will cost $72,714 a year.
More Coverage
Originally published as Promising new Alzheimer’s drug Aducanumab awaits TGA approval




