Restrictions that threatened doctors with a $13000 fine if they prescribed a certain drug revoked
A ban on doctors prescribing an ‘off label’ drug that patients seek for unapproved treatment of Covid-19 has been revoked by the Queensland chief health officer.
QLD News
Don't miss out on the headlines from QLD News. Followed categories will be added to My News.
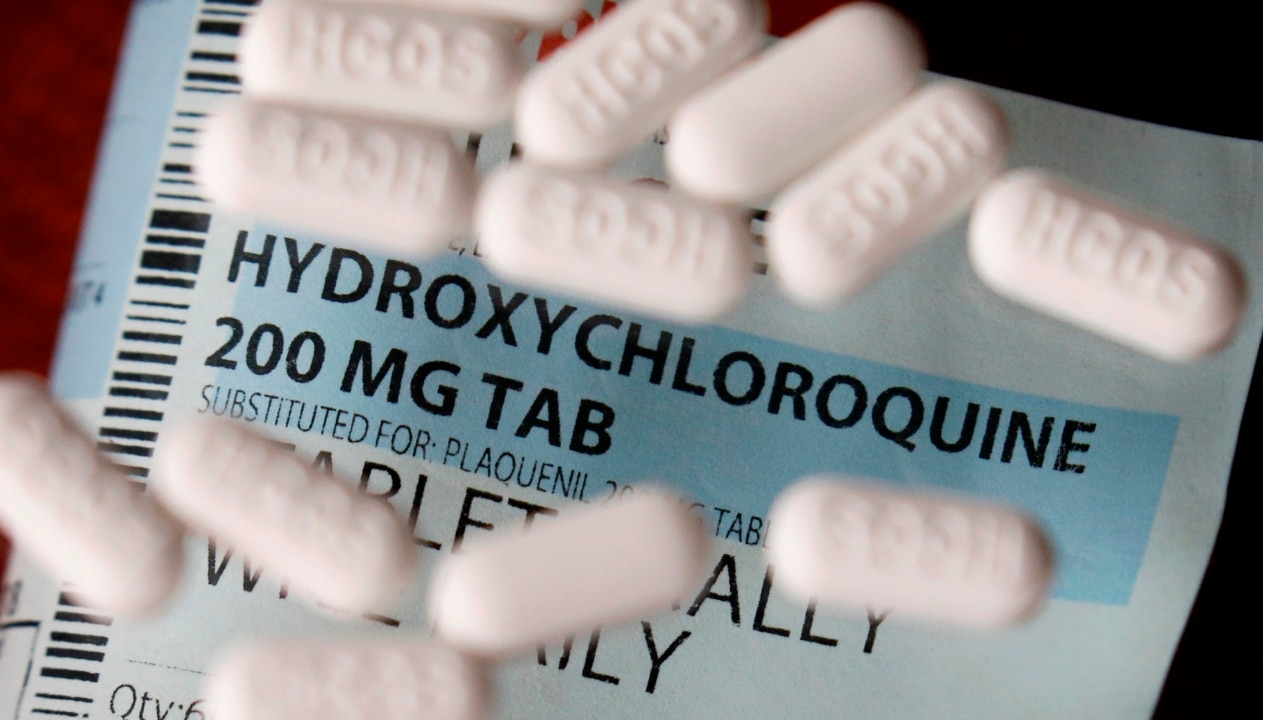
A Queensland Health order that threatened general practitioners with a $13,000 fine if they prescribed an antimalarial drug has been revoked.
Doctors were banned from prescribing, dispensing or supplying hydroxychloroquine in April 2020 after government officials claimed some people were asking their GP’s for the medication as an ‘off label’, or unapproved, treatment for Covid-19.
The increasing number of requests led to concerns there would be a supply shortage, leaving those already prescribed the drug for other, unrelated ailments without their medicine.
A March 12 update to the “Revoked and superseded public health directions” section of the Queensland Health website shows Chief Health Officer (CHO) Dr John Gerrard revoked the 2020 direction.
“I, Dr John Gerrard, Chief Health Officer, reasonably believe it is necessary to give the following direction pursuant to the powers under s362B of the Public Health Act 2005 to assist in contain, or to respond to, the spread of Covid-19 within the community,” he wrote.
In April 2020, then-CHO Dr Jeannette Young banned the use of the drug, except for those already on it for non-Covid-19 ailments, in accordance with emergency powers arising from the declared public health emergency – or the Covid-19 pandemic.
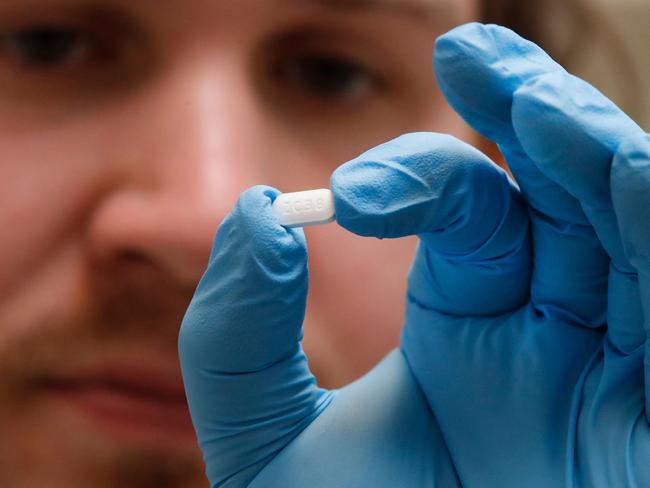
The restrictions meant only certain specialists were able to prescribe the medication.
Once banned, clinicians, other than the approved specialists, caught prescribing, supplying or dispensing hydroxychloroquine faced a $13,000 fine under the Public Health Act 2005 (Qld).
Although the use of the drug as a possible coronavirus treatment has been the focus of ongoing clinical studies worldwide, it is not approved for use by the Therapeutic Goods Administration.
The Commonwealth’s National Covid-19 Clinical Evidence Taskforce continues to strongly recommend people do not use hydroxychloroquine to treat Covid-19.
A Queensland Health spokeswoman said the revocation of the ban was because there was no longer a supply shortage threat, but the medication was still not approved for Covid-19 treatment.
“Early on in the pandemic, there was considerable focus on the potential for hydroxychloroquine to help in treating Covid-19,” she said.
“This resulted in high demand for the product and created concerns about potential shortages for patients already prescribed it for chronic conditions.
“Hydroxychloroquine is typically used for treatment of malaria and certain auto-immune diseases.”

The spokeswoman said the April 2020 restrictions were implemented in line with TGA advice issued in March that year, which ensured the medicine’s continued availability for patients prescribed it for auto-immune conditions and for use in approved clinical trials.
The March 2020 TGA report said the ban came about because some people were seeking the drug from clinicians as an unapproved or “off label” treatment for Covid-19.
As a result, it also created a supply shortage for those already on the medicine for other ailments such as some types of arthritis.
“Recent reports of increased off-label prescribing of medicines containing hydroxychloroquine have raised concerns that this will create a potential shortage of this product in Australia,” the report stated.
The 2020 report also said the medicine had potential serious side effects and discouraged its use for Covid-19 other than in the controlled trials taking place.
“Clinical trials are under way around the world examining their potential to treat COVID-19. However, these medicines pose well-known serious risks to patients including cardiac toxicity (potentially leading to sudden heart attacks), irreversible eye damage and severe depletion of blood sugar (potentially leading to coma),” it stated.

“Given the limited evidence for effect against COVID-19, as well as the risk of significant adverse effects, the TGA strongly discourages the use of hydroxychloroquine outside of its current indications at this time other than in a clinical trial setting or in a controlled environment in the treatment of severely ill patients in hospital.”
The TGA has been contacted for comment but are yet to respond.
The TGA, part of the Australian Government Department of Health, is responsible for regulating therapeutic goods including prescription medicines, vaccines, sunscreens, medical devices, vitamins and minerals and blood and blood products.
Many claim the uptake in people asking for the medication was at least in part because of high profile politicians, with no-known medical or scientific training, touting the drug as a possible novel coronavirus treatment.
In March 2020, then US president Donald Trump spruiked hydroxychloroquine as a possible treatment for the novel coronavirus, in contradiction to the advice given by US’s top public health officials and agencies at the time.

Australian businessman and former MP Clive Palmer also imported about 33 million doses of the medication into Australia, through his Palmer Foundation, in April 2020, saying he had donated the stockpile to the Federal government, should it be proven to be an effective treatment for Covid-19.
Mr Palmer later claimed, in several media interviews and paid advertisements, that Australia’s Covid death rate had flattened because of the use of the drug but those claims were proven to be unfounded.
In 2020 and 2021 respectively, the TGA gave provisional approval to medications called Remdesivir, also known as Veklury and Sotrovimab, also known as Xevudy, to treat Covid-19.
Oral medicine provisionally approved by the TGA this year include molnupiravir, also known as Lagevrio, and nirmatrelvir with ritonavir, also known as Paxlovid.
In February, provisional approval was given to tixagevimab and cilgavimab, also known as Evusheld, for the prevention of COVID-19 in people who are at risk of infection but have not been exposed to the virus.
Originally published as Restrictions that threatened doctors with a $13000 fine if they prescribed a certain drug revoked


