Some of the most popular breast implants to be banned over cancer link
Textured breast implants linked to a rare form of cancer were banned in Europe in June - and now Australia’s medical regulator is moving to ban them here.
Breast implants linked to a rare form of cancer could be cancelled and others suspended within weeks after a review by the nation’s medical watchdog.
The TGA today announced it was moving against textured implants widely used in discount breast clinics around Australia.
However, it is not recommending women who already have the implants get them removed.
There have been 81 cases of the rare blood cancer ALCL linked to the breast implants and three deaths in Australia.
Worldwide around 500 cases of the ALCL cancer have been linked to breast implants and there have been 16 deaths.
Experts estimate the risk of breast implant associated lymphoma is rare at between 1-in-1000 and 1-in-10,000, the watchdog said.
One of the most popular breast implants used in Australian women was pulled from the market in Europe in June.
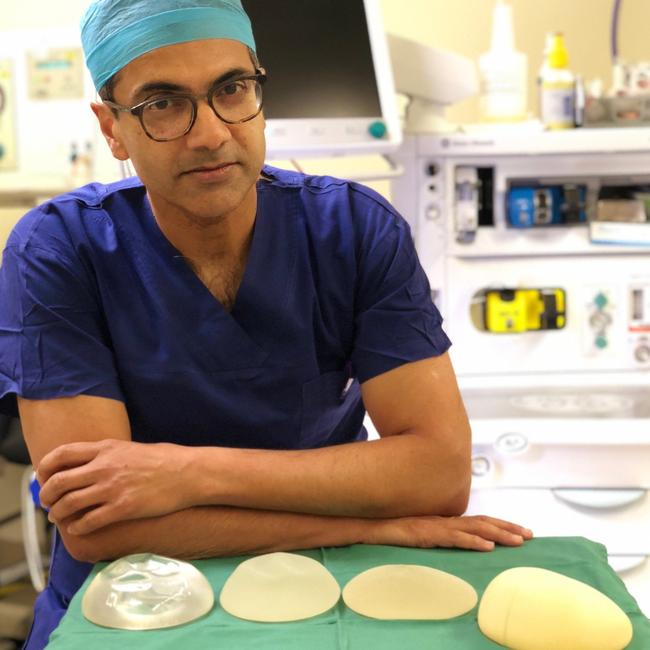
Today the TGA announced it was planning to cancel the same implant made by manufacturer Allergan. Its Natrelle implants and tissue expanders are in line to be cancelled from July 24.
Eight other brands could have their sales suspended including Nagor textured implants, Emagen, Emergo tissue expanders, Euro Cristaline, Poytech polyurethane implants, Johnson and Johnson Siltex implants and Mentor tissue expanders.
Around four in ten of the 40,000 women a year who have breast implants in Australia are fitted with Allergan’s natrelle or Biocell implant.
The sponsors of the devices have until July 24 to respond to the proposed bans.
News Corp has been campaigning for the companies to pay for the removal of the devices in cancer cases, for all breast devices to be recorded on a device registry and for it to be made mandatory for surgeons to check women with the implants every 12 months.
Health Minister Greg Hunt said in a statement breast implant associated lymphoma is usually cured if detected early.
“If you have concerns about your breast implants, see your general practitioner and seek review by your surgeon,” he said.
The Government has set up an Australian Breast device registry to monitor outcomes for patients with breast implants.
It also provides funding through Medicare for the treatment of complications resulting from breast implants.
“The Morrison Government supports access to quality, safe and effective health care and actions from regulators that are in the interest of public health and safety,” Mr Hunt said.
Implant manufacturer Allergan said “patient safety remains Allergan’s highest priority” and the company said it “continues to stand behind the benefit/risk profile of its breast implants, including Biocell textured breast implant products”.
“With every medical procedure, there are benefits and risks, and Allergan continues to believe the benefit/risk profile of Biocell textured breast implant products remains positive.”
“There continues to be no recommendation from any health authority, including the TGA, for asymptomatic patients to have their textured breast implants removed or replaced prophylactically,” it said.
The Morrison Government is providing $48.2 million over 2017-18 to 2022-23 to the McGrath Foundation for breast cancer nurses. This includes increasing the number of Commonwealth-funded McGrath Breast Care Nurses from 57 to 98 by 2022-23.
In the 2019-20 Budget, the Government invested $32.6 million over four years to list additional magnetic resonance imaging (MRI) and positron emission tomography (PET) items on the Medicare Benefits Schedule for the detection and evaluation of breast cancer. It is expected that this will support around 14,000 patients each year.
The Morrison Government supports access to quality, safe and effective health care and actions from regulators that are in the interest of public health and safety.
The TGA has published information on the proposed regulatory action, including information on the specific textured implants, on the TGA breast implant hub https://www.tga.gov.au/hubs/breast-implants.
Australian patients with questions or concerns about Biocell textured breast implant products should, in the first instance, consult with their healthcare professional. Patients and healthcare professionals can also contact Allergan’s Medical Information team at medinfo.australia@allergan.com or by phone at 1800 252 224.


