Countries that have approved and are rolling out booster vaccinations
Which countries have approved and are already rolling out booster vaccines and which vulnerable demographics have access to the jabs?
Industrialised and richer nations including the United States, the United Kingdom, Israel, and the European Union soon to follow, have already vaccinated a major proportion of their populations, but have already started or announced programs to offer fully vaccinated people an extra dose of Covid-19 vaccine. The rising number of breakthrough cases of coronavirus are increasing the urgency with these nations rolling out millions of booster jabs. Here’s what they are offering and to whom.
US
Approximately 60 million people in the United States are now eligible for a Pfizer booster shot against Covid-19, US President Joe Biden said on Friday 24 September as a regulatory marathon laying bare divisions within the scientific community on the issue came to a close.
As the US announced the rollout of its Covid-19 booster shots it revealed that this third phase of inoculation will rely on the honour system.
“As of today, up to 20 million Americans can get their booster shots,” Jeff Zients, the Biden Administration’s head of coronavirus, said at a White House briefing.
“We have been preparing and we are hitting the ground running to get booster shots in arms,” he continued.
“There are people who will be getting booster shots as early as this afternoon,” he said.
The move makes a third dose available to Americans aged 65 and over, people over 50 with chronic health issues, and those at high risk due to their jobs or living conditions — such as healthcare workers and teachers, or those who live in mass housing such as homeless shelters or are in jail.
The boosters can be administered six months after the recipient’s last jab.
The rollout will rely on “self-attestation” — or the honour system — to prevent non-eligible people from getting boosters, added CDC Director Rochelle Walensky.
The booster shot launch comes after Ms Walensky made the unusual decision to go against a CDC advisory panel which recommended that boosters be allowed for Americans with high-risk jobs and living conditions in addition to those over 65 and people 50 and older with underlying health issues.
Her endorsement approved booster shots for millions more Americans and aligned with the Food and Drug Administration’s authorisation earlier last week.

In the end, US health authorities have recommended boosters for three categories of people: those 65 and older, those 18-64 with an underlying medical condition such as diabetes or obesity, and those who are especially exposed to the virus because of their work or where they live.
A total of 20 million people got their second Pfizer shot at least six months to qualify now for a booster, Mr Biden said.
“Go get the booster,” he said in remarks at the White House. “I’ll be getting my booster shot,” the 78-year-old president added, “as soon as I can.”

Which vaccine?
The US booster rollout is with Pfizer/BioNtech.
Mr Biden said people who have received Moderna or Johnson & Johnson vaccinations could get booster shots once studies have been completed and he expected that all Americans would be eligible “in the near term.”
Ms Walensky said data on Moderna and Johnson & Johnson booster shots would be evaluated “in the coming weeks.”
Some immunocompromised people in the United States have been eligible to receive a third dose of the Pfizer or Moderna vaccine since early August.

UK
The woman who became the first person in the world to get the Pfizer Covid-19 vaccine has had her booster jab.
Margaret Keenan, 91, from Coventry, England received her third injection at University Hospital in the city on Friday 24 September, which was at the same place she was first vaccinated.
Also getting the booster was Matron May Parsons, who administered Ms Keenan’s first jab back in December 2020,
Ms Keenan said she felt happy she had got it done and that it meant she felt free.
The UK has administered more than 48 million first doses of Covid-19 vaccine so far.
Booster shots are now being offered to the over-50s, younger adults with underlying health conditions and frontline healthcare workers.
The recommendation came from Joint Committee on Vaccination and Immunisation to the government of Boris Johnson amid concern about waning immunity of the vaccines and a spike in breakthrough infections.
On 13 September, Mr Johnson announced that he was backing booster shots for vulnerable people and the elderly as part of his plan to prevent hospitals being overwhelmed as the UK approaches winter.
The UK will roll out booster shots for what will amount to around 30 million people, including for children between the ages of 12 and 15 to help stop Covid-19 spreading in schools, which have now resumed fully.
Which vaccine?
A half dose of the Moderna vaccine may be used, as this has been shown to be very effective. The Pfizer, Moderna and Oxford/AstraZeneca vaccines have all been approved for use as booster jabs by the UK medicines regulator, the MHRA.
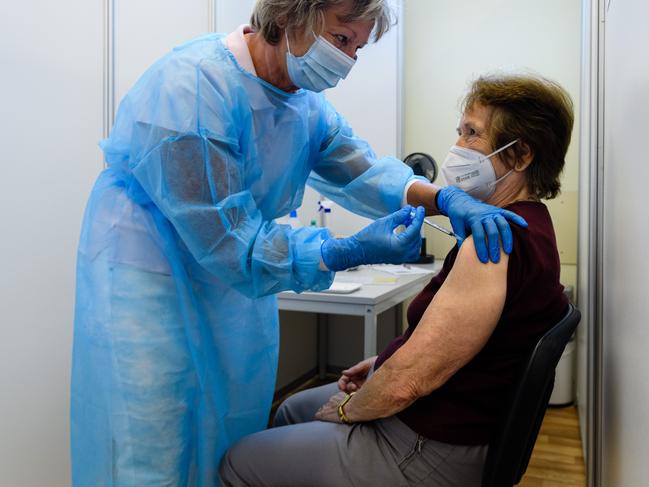
EU
The European Medicines Agency (EMA) is on the brink of deciding whether to endorse a third dose of the Pfizer-BioNTech to be given to those who had their second dose six months ago.
“The outcome of this evaluation is expected in early October, unless supplementary information is needed,” EMA’s head of vaccines strategy, Marco Cavaleri, told a press briefing.
Mr Cavaleri’s statement confirmed a Reuters report earlier in the day on EMA’s expected review time on the matter.
EMA added that, in early October at the latest, it would conclude its review of the use of both the Pfizer-BioNTech and the Moderna shots in people with a weak immune system already one month after their initial two-shot regimen.
In an opinion issued in early September and republished by the EMA, the European Centre for Disease Prevention and Control said there was no urgent need to administer booster doses to fully vaccinated people in the general population.
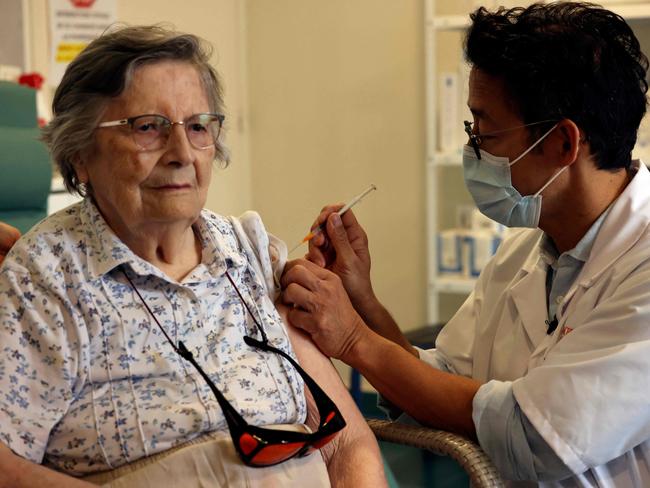
But many EU states including Germany, France, Spain, and the Czech Republic have already decided to administer a booster dose despite facing higher legal risks without a formal decision to do so by the EMA.
EMA has conceded there may be merits in doing so.
“With an increase in breakthrough cases that we’ve seen over time, we do understand that member states in Europe and countries elsewhere want to consider now the option of a booster, particularly in vulnerable groups and that is why we have been expediting our review,” said Mr Cavaleri.
Which vaccine?
The EU regulator said on 6 September it had begun its evaluation of data submitted by Pfizer and BioNTech for a booster dose to be given six months after the second dose in people 16 years of age and older.
Moderna is also expected to submit data to the EMA this month on its booster dose, an EU document said.
The EU has signed three deals with Pfizer and BioNTech for a total of 2.4 billion doses.
The latest contract covers the supply of at least 900 million shots, a large part of which is likely to be needed only if boosters are considered necessary, or if new virus variants emerge against which existing vaccination is not effective.
Over 70 of the EU’s adult population has already been fully vaccinated, and the bloc has secured an ample supply of vaccines from several manufacturers.

ISRAEL
After having the world’s fastest vaccination program and believing they had beaten the virus, Israel suffered rising infections once mask mandates were removed and lockdowns lifted - despite a majority of the population being vaccinated.
To try and contain a surge in coronavirus cases, authorities in August began administrating a booster shot to those aged 50 and older, after starting a campaign for over-60s late last month.
The state lowered the age threshold to receive a third coronavirus booster dose to anyone aged 30 and above, as it continues to battle surging infections.
Israel is now offering boosters to vaccinated people as young as 12.
In late August, the government made booster shots available to anyone at least five months after their second jab.

Which vaccine?
Israel has mainly used the Pfizer-BioNTech vaccine in its rollout, and started offering booster doses of the same as early as July to people over 60 years old.
Israel so far has administered more than two million booster shots, and the government has said it is preparing to ensure it has sufficient supplies in case a fourth dose is needed.
More Coverage
Originally published as Countries that have approved and are rolling out booster vaccinations




