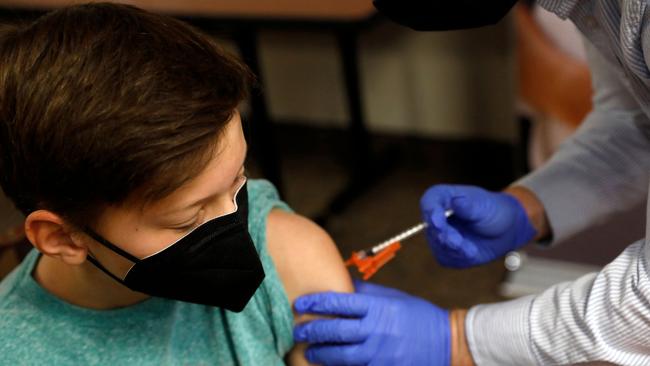
Coronavirus world: US approves use of Pfizer vaccine in children aged 5 to 11
Young children could soon get Pfizer’s Covid-19 vaccine in the US, after the Food and Drug Administration approved it for “emergency use”.
Children aged 5 to 11 could soon get Pfizer’s Covid-19 vaccine in the US, after the Food and Drug Administration approved it for “emergency use”.
The FDA cleared doses for children — a much smaller amount than that given to adults. It means up to 28 million more American children could be eligible for vaccinations as of next week, according to the New York Post.
The Centre for Disease Control and Prevention is due to make a more detailed recommendation on which children should get vaccinated against Covid next week.
“With this vaccine kids can go back to something that’s better than being locked at home on remote schooling, not being able to see their friends,” Dr Kawsar Talaat, of Johns Hopkins University, said, per the New York Post.
“The vaccine will protect them and also protect our communities.”
Some others countries have begun using Covid-19 vaccines in children under 12, including China, which has started vaccinating 3-year-olds.
COVID VACCINE ‘PILL’
South Africa has approved a clinical trial for a Covid vaccine pill, Israeli pharmaceutical company Oramed said.
Its majority-owned subsidiary Oravax Medical has received clearance to begin enrolling patients in the trial of an oral vaccine, the company said in a statement.
South Africa has hosted several Covid vaccine trials. This will be the first for an oral approach.
Oral vaccines are particularly attractive for the developing world because they reduce the logistical burden of immunisation campaigns, the company said.
But they could also increase uptake in wealthy countries where needle aversion is an often missed factor in hesitancy.
“They do not require a medical professional to administer,” the company said in a statement. “They are more desirable to the potential recipients because they do not involve injections.” A vaccine pill could become especially attractive if ongoing boosters are required.
The World Health Organization also on Thursday warned that Africa’s vaccination efforts risked being paralysed by a severe shortage of syringes needed to administer jabs.
$4 DRUG COULD REDUCE COVID SEVERITY
A cheap and widely available antidepressant medication, meanwhile, may join a growing list of Covid-19 treatments after it showed promising signs in reducing hospitalisations and deaths, according to a new study.
The study, published in the Lancet journal, found that giving high-risk Covid patients fluvoxamine — 100mg twice daily for 10 days — early in their treatment, cut the length of patients’ hospital stays by up to a third.
Fluvoxamine, which is produced by several manufacturers and sold under the brand name Luvox, is often prescribed off-label for major depressive disorder, post-traumatic stress disorder, eating disorders and other conditions.
“Fluvoxamine may reduce the production of inflammatory molecules called cytokines, that can be triggered by SARS-CoV-2 infection,” Associate Professor of Psychiatry at Washington University, Dr. Angela Reiersen, said.
The clinical trial took place from January to August and included roughly 1,500 people at 11 sites in Brazil. The participants were adults who were symptomatic with Covid-19 and at heightened risk of severe illness because of other health problems.
Researchers also found that the $4 drug may reduce blood platelets, which may affect the clotting effects of coronavirus infection.
And while they admitted this finding would not be a cure to the virus, they added it would be useful if the drug kept patients out of the hospital.
“Given fluvoxamine’s safety, tolerability, ease of use, low cost, and widespread availability, these findings might influence national and international guidelines on the clinical management of Covid-19,” they concluded.
The researchers said that they would also be looking also into another cheap and widely available drug Prozac, or fluoxetine, to see if it could also help Covid-19 patients.
“It is now crucial to establish whether a class effect exists and whether these drugs can be used interchangeably for Covid-19,” they wrote.
“There is no standard of care that exists for early treatment of COVID-19 and various advocacy groups promote different interventions, including some of those evaluated in this and our previous trials.
“Furthermore, there is little understanding of who is at greatest risk of disease progression from this disease as some patients with numerous risk factors do recover quickly whereas some others with less established risk factors might note.”
US expert says vax protection in kids ‘over-estimated’
The only member of a US government medical panel not to endorse the Covid vaccine for children says Pfizer “over-estimated” its benefits, which do not outweigh the risks to many kids.
The Food and Drug Administration’s panel of independent experts voted 17 to 1 abstention in favour of approving the vaccine for five-to-11-year-olds.
Dr Michael Kurilla, who abstained from the vote, said in a statement that Pfizer “likely over-estimated overall ‘infection’ prevention” since the clinical trial of just 2,268 kids only evaluated symptomatic diseases.
The trial did not test for infection, only for symptoms. That is despite half of all children not showing any symptoms from a Covid infection. And the younger the child, the less likely they are to show symptoms, while those that do show symptoms rarely result in severe disease
“While there are clearly high-risk groups within the 5 - 11 age group for which this vaccine would significantly reduce serious disease, I do not expect protection from infection to last more than a few mon
this and this may negatively impact public perception of vaccines,” he said in a statement.
Given that about 40 per cent of children already have a Covid infection, giving them some natural immunity, emergency use authorisation wasn’t necessary for the entire age group, the director of the Division of Clinical Innovation at the National Institutes of Health’s (NIH) National Center for Advancing Translational Sciences added.
Dr Kurilla said in a follow-up interview with The Daily Mail that he abstained rather than voted “no” to not undermine confidence in the vaccine.
“Does vaccinating [kids who have previously been infected] provide additional protection? Probably,” he told the Mail.
“Will it hurt them? Probably not. Will it help them? Maybe, but I don’t think there’s any additional benefit. I don’t think the benefits outweigh the risks.”
US PANEL ENDORSES PFIZER VAX FOR KIDS
The vote by Dr Kurilla’s colleagues paves the way for 28 million children to get their shots as early as next week.
The independent experts concluded the known benefits, both directly to kids’ health but also in ending school and other disruptions, outweighed the known risks.
The Food and Drug Administration, which convened the meeting, is expected to give its formal green light soon.
“It is pretty clear to me that the benefits do outweigh the risk when I hear about children who are being put in the ICU, who are having long term outcomes after their Covid, and children are dying,” said Amanda Cohn of the Centers of Disease Control and Prevention (CDC), who voted yes.
“It’s never when you know everything -- the question is when you know enough,” said Paul
Offit, a paediatrician at the Children’s Hospital of Philadelphia, who also voted yes but reflected on the fact that more complete safety data would become available over time.
He added that many children who are at high risk stand to benefit, and that the theoretical risk of myocarditis, the most worrisome side-effect, would probably be very low, given the lowered dose of 10 micrograms, compared to 30 micrograms in older ages.
Nevertheless, several experts partly caveated their votes by saying they would not favour broad vaccine mandates in schools and the shot should remain a personal decision for families.
Earlier, top FDA vaccine scientist Peter Marks said younger children were “far from being spared harm of Covid-19,” adding that, in this group, there had been 1.9 million infections and 8,300 hospitalisation, roughly a third of which required intensive care.
There have also been around 100 deaths, making it a top 10 leading cause of death, he added.

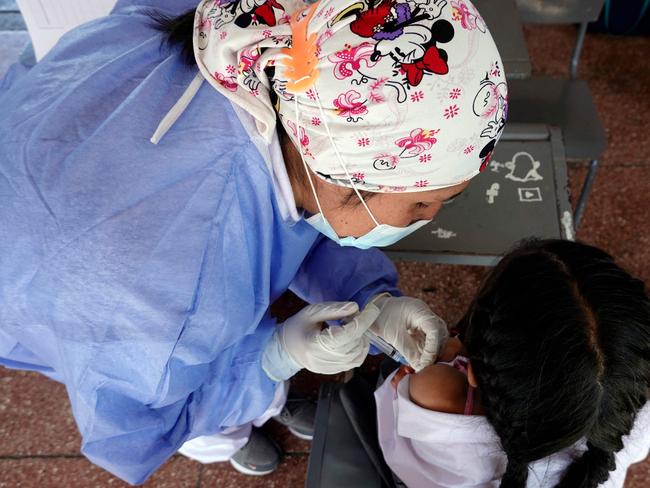
CHINA STARTS VACCINATING TODDLERS
China, meanwhile, has already started vaccinating children as young as three as part of clampdown measures to maintain a zero-tolerance policy towards outbreaks.
Local city and provincial level governments in at least five provinces issued notices in recent days announcing that children aged three to 11 will be required to get their vaccinations, AP reports.
The shots, developed by homegrown drugmakers Sinovac Biotech Ltd and state-owned Sinopharm, have already been administered to those aged 12 and above, with the country green-lighting their use in those aged over three in June, according to Bloomberg.
Gansu, a northwestern province heavily dependent on tourism, closed all tourist sites on Monday after finding new Covid cases.
Residents in parts of Inner Mongolia have been ordered to stay indoors due to an outbreak there.
The National Health Commission reported 35 new cases of local transmission had been detected over the past 24 hours, four of them in Gansu.
Another 19 cases were found in the Inner Mongolia region, with others scattered around the country.
China has employed lockdowns, quarantines and compulsory testing for the virus throughout the pandemic and has largely stamped out cases of local infection while fully vaccinating 1.07 billion people in its population of 1.4 billion.
About 76 per cent of the country’s 1.5 billion people have been fully vaccinated.
The country is now rolling out booster shots, with adults who had their first doses six months ago now eligible.
MODERNA JAB ‘SAFE FOR KIDS’
US biotech firm Moderna said Monday local time its Covid vaccine was safe and produced a strong immune response in children aged 6-11, adding it would submit trial data to global regulators soon.
The news comes as a panel of government advisers was preparing to meet Tuesday on the question of whether to authorise the Pfizer vaccine in kids aged 5-11, with top infectious disease expert Anthony Fauci predicting it would be available by mid-November.
“We are encouraged by the immunogenicity and safety profile of mRNA-1273 in children aged 6 to under 12 years and are pleased that the study met its primary immunogenicity endpoints,” Moderna chief executive Stephane Bancel said in a statement.
An interim analysis from a mid-to-late stage clinical trial of 4,753 children showed that two doses of vaccine produced a high level of neutralising antibodies — Y-shaped proteins that bind to the coronavirus and block it from entering human cells.
The vaccine was dosed at 50 micrograms, which is half of what is used among adults, but still produced on average 1.5 times as many antibodies in children as it did in young adults given the higher dose.
The majority of adverse events were mild or moderate, including fatigue, headache, fever, and injection site pain.
These early results, released via a press statement, do not yet include a vaccine efficacy estimate, which may be expected at a later time once cases have accrued.
PFIZER: VAX FOR YOUNGER KIDS 90 PER CENT EFFECTIVE
Pfizer’s Covid-19 vaccine is more than 90 per cent effective in preventing symptomatic disease among children aged 5-to-11, the company said in a document released Friday that put forward its case for authorisation.
The new data was published on the website of the Food and Drug Administration, which has called an advisory panel of independent experts to meet Tuesday to vote on whether to green light the shot.
The analysis was based on around 2,250 trial participants, randomised to receive either the vaccine or a placebo, with data accruing until October 8. Most positive cases occurred when the Delta variant was dominant in the US and globally.

“VE (Vaccine Efficacy) against laboratory-confirmed symptomatic Covid-19 occurring at least 7 days after Dose 2 in evaluable participants without evidence of prior SARS-CoV-2 infection was 90.7 per cent,” the document said.
The vaccine was tested with a 10 microgram dose, while older age groups have received 30 micrograms. The doses were administered three weeks apart.
There were no cases of severe Covid and no cases of multisystem inflammatory syndrome in children (MIS-C), a rare but serious post viral condition.
Overall, 158 children aged 5 to 11 have died from Covid in the United States since the start of the pandemic, according to official data.
Pfizer argued that “although the mortality rate for Covid-19 in children is substantially lower than that in adults, Covid-19 was among the top 10 leading causes of death for children 5 to 14 years of age between January and May 2021 in the US.”
Safety data was examined among a total of 3,000 vaccinated participants, with a low incidence of severe events.
There were no cases of myocarditis or pericarditis — inflammation or inflammation around the heart — but there were not enough study volunteers to be able to detect highly rare side effects.
In any case, male adolescents and young men are thought to be a higher risk group for these conditions.
This is the first time Pfizer has released an efficacy estimate for its Covid vaccine in younger children, along with a detailed dataset.
Its earlier press statements only said the vaccine produced a robust immune response and was safe.
Throughout the pandemic, pharmaceutical companies have been making major announcements through press releases with scant data, a situation that has frustrated some experts.
A document containing the FDA’s own analysis should be released soon, and will give an indication of the agency’s own view on whether the benefits of the vaccine outweigh the risks for this group.
The administration of President Joe Biden has said it stands ready to roll out shots for the country’s 28 million 5 to 11-year-olds as soon as the vaccine is authorised by science agencies.
The FDA panel meeting will be followed by a panel convened by the Centers for Disease Control and Prevention (CDC) on November 2-3. If both committees vote in favour, authorisation could follow within days or weeks.
Originally published as Coronavirus world: US approves use of Pfizer vaccine in children aged 5 to 11


