Sydney family’s plea for life-saving cancer treatment
“After the children have been through so much, to deny them this is just so cruel.”
Family Life
Don't miss out on the headlines from Family Life. Followed categories will be added to My News.
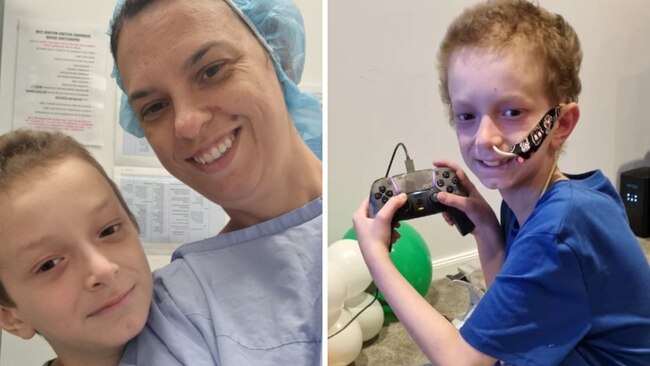
Harry was only eight years old when his life changed forever.
A month before his ninth birthday, the Sydney boy started complaining of stomach aches; the once active kid, who loved playing outside, no longer had the energy to play soccer.
After weeks of testing, the family learned the devastating diagnosis: Stage 4 High-Risk Neuroblastoma.
Want to join the family? Sign up to our Kidspot newsletter for more stories like this.

“It was a tumour; it was in his bone; it was everywhere”
“Every time he got a test, it got worse,” mum Julia told A Current Affair.
“It was a tumour; it was in his bone; it was everywhere. The cancer was everywhere.”
RELATED: 'My daughter thought it was just a chocolate stain ... she saved my life'
Although classified as a rare cancer, Neuroblastoma is the most common childhood tumour, with roughly 50 children diagnosed with it each year.
According to Neuroblastoma Australia, the average age of diagnosis is just two years old.
With a 50 per cent survival rate, it’s also one of the deadliest cancers in the country, claiming the lives of more children under the age of five than any other cancer.
Introducing our new podcast: Mum Club! Listen and subscribe wherever you get your podcasts so you never miss an episode.
Following the devastating diagnosis, Harry immediately began treatment for neuroblastoma.
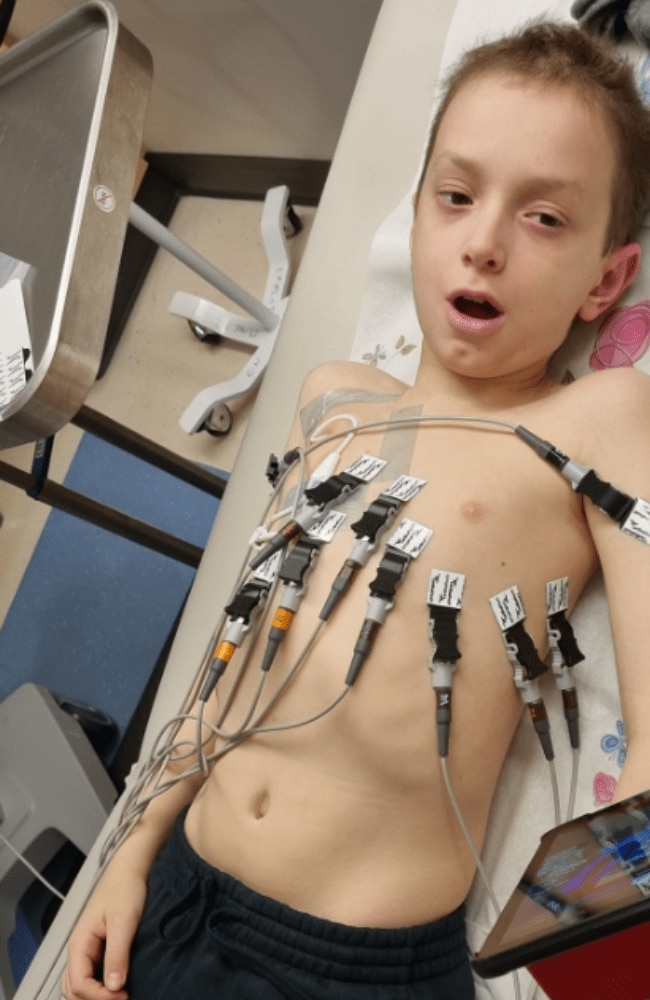
Over the span of 18 months, the family’s world was turned on its head, with Harry undergoing surgery to remove a tumour and two tandem bone marrow transplants, followed by 12 rounds of immunotherapy and radiotherapy and six rounds of chemotherapy.
Despite all the medication and therapy he’s undergoing, there’s a 50 per cent chance he could relapse, and if he does, there’s only a five per cent chance of survival.
However, one treatment may help Harry in his fight. The only issue is that it’s not available in Australia.
RELATED: 'He’s my hero': Adelaide 8yo’s final wish of becoming a police officer is granted
“Children with neuroblastoma don’t have the time for rare diseases”
In December, Harry and his family made the first of five trips to the US, where they can access the potentially life-saving drug.
The journey to the US isn’t simple or cheap, with the family saving every cent they can to make the 18-hour flight to Pennsylvania, where he undergoes DFMO treatment.
Difluoromethylornithine, better known as DFMO, is a drug that blocks a molecule in the body that helps cancer cells spread. When combined with traditional methods like chemotherapy, it can significantly reduce the likelihood of relapsing.
The medication was approved by the US Food and Drug Administration (FDA) in December 2023, after showing promising signs for children diagnosed with the deadly cancer.
Now, Neuroblastoma Australia is urging the TGA to approve the drug in Australia to prevent other children from suffering.
Under the Therapeutic Goods of Australia Special Access Scheme, individual Australian families are able to access the DFMO drug. However, it can cost anywhere from $500,000 to $700,000 for treatment, far out of the budget of most households.
Speaking to ACA, Lucy Jones, chief executive of Neuroblastoma Australia, wants the federal health minister Mark Butler to fast-track approval “to help all these beautiful Neuroblastoma children access the drug while they can to save their life".
“Children with neuroblastoma simply do not have the time for rare diseases,” she said. “The regulatory approval process needs to be quicker.”
Ms Jones believes the federal government would have to spend between $5 million and $15 million over a two-year span to approve the DFMO drug in Australia, which roughly accounts for “0.1 percent of the federal budget.”
RELATED: 'She wanted to tell us she loved us one last time before she passed'
In response to Neuroblastoma Australia’s request, health minister Mark Butler confirmed he had written to pharmaceutical company Norgine, urging them to register the life-saving drug in Australia.
You can read his full statement below:
“These stories are heartbreaking.
Neuroblastoma is a highly aggressive cancer often found in children with approximately 40 new child diagnoses each year in Australia.
Despite intensive treatment, the survival rate is only 50 per cent.
US Food and Drug Administration has recently approved a drug called difluoromethylornithine (DFMO) that has demonstrated excellent results in clinical trials.
Recently, the TGA granted DFMO Orphan Drug Designation which incentivises the sponsor to make the medicine available in Australia by exempting the company from a range of fees that normally apply to registration.
Pharmaceutical companies can also consider providing their product to these families through their compassionate access program.
I wrote to Norgine encouraging them to make an application to the TGA to register DFMO in Australia and to consider compassionate access to Australian families in the meantime and consider options to expand clinical trials in Australia.
Norgine replied recognising the severe unmet medical need in the treatment in Australia, and is committed to providing equity of access to all patients who are eligible for DFMO.
Norgine have now lodged an application for DFMO with the TGA. They are now working with the TGA to achieve this as quickly as possible.”
More Coverage
Originally published as Sydney family’s plea for life-saving cancer treatment





