Five words after surgeon’s shocking error revealed
The five words said by a doctor while standing over a woman’s hospital bed following surgery changed her life forever.
Health
Don't miss out on the headlines from Health. Followed categories will be added to My News.
The five words said by a doctor while standing over a woman’s hospital bed following surgery changed her life forever.
Teresa Burbery, from Brisbane, had undergone a minor surgery in September 2021 after a bike accident left her in chronic pain.
She’d been through multiple treatments to try and ease her pain and decided to go to a new clinic. There, she was told by the pain specialist who would operate on her that a spinal cord stimulator would ease her pain by 60 to 70 per cent.
A spinal cord stimulator is a small device that sends small electrical pulses near the spinal cord to ease pain.

“I heard that and I thought, ‘My god, that’s life-changing. That’s me being able to go running again’,” Teresa told ABC’s Four Corners for part of its investigation into the dangers of the devices.
Teresa went through with the surgery but as she started to wake up she realised something wasn’t right. The specialist was standing over her.
“I’m so sorry Teresa, I’m so sorry Teresa … I’ve struck your spinal cord,” the specialist told her.
Her left leg felt heavy and she began to cry out in pain.
Teresa ended up having to stay in hospital for six weeks following what was supposed to be a minor surgical procedure. She faces paralysis in her leg and needs to make use of a wheelchair.
She now requires three times the amount of pain killers than she did before and feels as though her specialist downplayed the risks of the procedure.
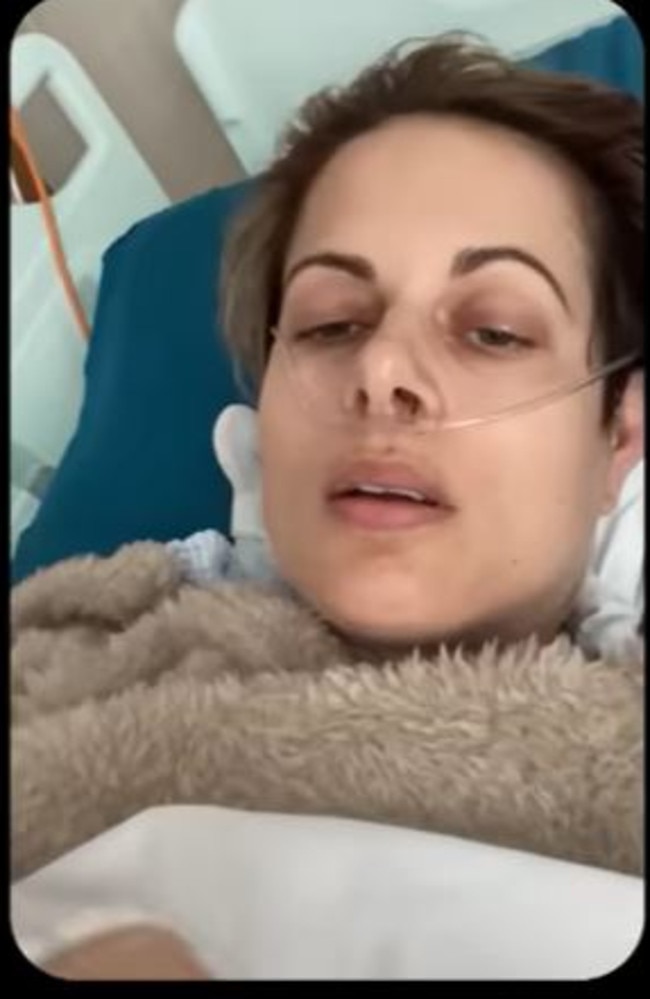
Teresa said she suffered side effects including loss of sexual, bladder and bowel function.
The Therapeutic Goods Administration, which approves devices and medications for use in Australia, said it had received 2000 reports of adverse impacts of the devices since 2012, and launched a post-market review in 2022. The results have not yet been published.
A spokesperson told news.com.au it takes the concerns seriously and that “patient safety remains our number one priority, and we strongly encourage any person with information about adverse events experienced by patients to report them directly to the TGA”.
“The TGA is investigating allegations made by 4Corners regarding under-reporting and will take regulatory action including civil or criminal penalties if appropriate,” the spokesperson said.
“The TGA is working as quickly as possible to finalise the post-market review investigations within the legislative guidelines. Our investigation includes 89 devices and our legislation requires the TGA to investigate each individual device separately.”
However, the Medical Technology Association of Australia has stood by the spinal cord stimulators saying each year 1200 privately insured Australian patients are implanted with them.

“As a nation, we must acknowledge Australia is in a chronic pain crisis. One in five Australians over the age of 45 are living with chronic persistent pain, yet according to Pain Australia, up to 80 per cent of people living with chronic pain are missing out on treatment that could manage their pain effectively including access to pain specialists and interdisciplinary care,” a statement on their website read.
It said that people have tried everything before they turn to the electrical devices.
“SCS has demonstrated the ability to produce clinically meaningful pain relief, improve quality of life, and significantly reduce the need for medication usage in individuals suffering from chronic pain,” the statement said.
“Today’s SCS devices offer the opportunity to tailor the pain management therapy to each patients’ circumstances. Importantly, technology is undergoing continual improvement through global clinical trials with therapy development and connected healthcare support.”

It said the methodology behind the meta-analysis provides a limited view of evidence and ignores other key data points.
“In order to truly assess long-term safety and effectiveness of a therapy the totality of the evidence must be considered,” it said.
“Importantly, MTAA members have actively participated in the TGA’s post-market review of spinal cord stimulation (SCS) devices. The purpose of the review was to reassess the safety and performance of these devices taking into consideration the additional real-world evidence associated with the use of SCS devices for Australian patients with chronic pain. We will continue to support the process to ensure these patients and their clinicians continue to have this clinical option available.”
Following the Four Corner’s episode, the Australian Physiotherapy Association (APA) polled its members and discovered 90 per cent say they have treated a patient who has come to them after harmful or unnecessary surgery.
Originally published as Five words after surgeon’s shocking error revealed





