Recce gets nod to progress Phase 2 trial for skin infections
A key body has unanimously agreed the Phase 2 trial of Recce’s lead compound for acute bacterial skin and skin-structure infections should continue.

Stockhead
Don't miss out on the headlines from Stockhead. Followed categories will be added to My News.
Non-DSMB unanimously agree Recce’s topical gel R327G is safe and well-tolerated in patients with acute bacterial skin and skin-structure infections
R327G demonstrates highly encouraging efficacy results with all patients completing treatment achieving either complete cure or improvement
No serious adverse events noted in patients with recommendation for clinical trial to continue
Special Report: A key body has unanimously agreed that the Phase 2 trial of Recce’s lead compound R327G in patients with acute bacterial skin and skin-structure infections (ABSSSI) should continue.
An independent non-Data Safety Monitoring Board (non-DSMB) has completed a positive review of safety and efficacy data from Recce Pharmaceuticals’ ongoing Phase 2 clinical trial of its lead compound, RECCE® 327 Gel (R327G), in patients with ABSSSI, including diabetic foot infections.
Recce Pharmaceuticals (ASX:RCE) noted that the non-DSMB review found no safety concerns and unanimously recommended continuing the clinical trial, on track to be completed within the calendar year.
The decision was based on R327G’s strong safety profile, with no serious adverse events observed in patients and highly encouraging efficacy results.
The open-label, pilot efficacy study is designed to evaluate the efficacy and systemic absorption of R327G when applied directly to the infected area.
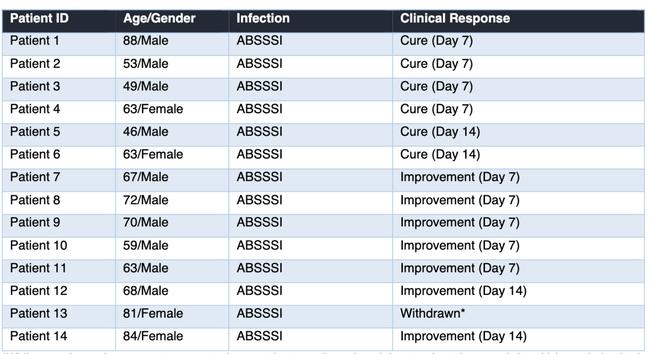
Most patients treated with R327G have demonstrated highly encouraging efficacy results, with all patients completing treatment positive on the primary endpoint and achieving either complete cure or improvement. Many showed complete cure results as early as seven days.
The outcomes were measured using the Lipsky Clinical Resolution of Infection Scale, a widely recognised tool for assessing the resolution of infections, particularly in diabetic foot infections.
Recognised by the FDA, RCE said the Lipsky Scale was an approved and reliable method for evaluating the treatment of wound infections.
While no serious adverse events were noted, RCE said one patient discontinued treatment due to pain at the wound site, which was judged to be unlikely related to R327G.
Findings further support safety of R327G
The non-DSMB's positive findings further underscore the strong safety profile of Recce’s innovative anti-infective therapy.
The conditions treated included diabetic foot ulcer, eczema, scratch and puncture wound infections.
A wide variety of infecting bacteria (gram positive and gram negative) were isolated and successfully treated with improvement/cure of infection in all patients that continued with their treatment.
"We're seeing some very promising results from the interim data in the Phase II trial, which confirm the safety and potential efficacy of R327G in treating Acute Bacterial Skin and Skin Structure Infections, including diabetic foot infections,” Coordinating principal investigator of the study Professor Eugene Athan said.
RCE CEO James Graham said the company was extremely encouraged by the feedback from the non-Data Safety Monitoring Board and the ongoing safety and efficacious profile of R327G.
“The absence of serious adverse events, coupled with the wide range of broad-spectrum efficacy across challenging wound infections, reinforces the potential of R327G to address unmet medical needs in the treatment of serious bacterial infections,” he said.
The Global ABSSSI treatment market size was valued at $7.3B USD in 2018 and is projected to reach $26B USD by 2032, representing a CAGR of 9.5% between 2019 and 2032.
This article was developed in collaboration with Recce Pharmaceuticals, a Stockhead advertiser at the time of publishing.
This article does not constitute financial product advice. You should consider obtaining independent advice before making any financial decisions.
Originally published as Recce gets nod to progress Phase 2 trial for skin infections


