Health regulator crackdowns on cosmetic injectables industry
The changes come after a slew of cosmetic injectable providers have come under fire for dodgy practices and “putting profit before patient safety”.

Lifestyle
Don't miss out on the headlines from Lifestyle. Followed categories will be added to My News.
Healthcare regulators in Australia have brought in new guidelines for dentists and nurses who perform non-surgical cosmetic injections, aiming to safeguard the public from practitioners who prioritise profits over patient safety.
On Tuesday, the Australian Health Practitioner Regulation Agency (AHPRA) released new rules requiring healthcare practitioners to undergo additional education and training before conducting cosmetic procedures such as botulinum toxin injections (Botox) and filler injections.
They also introduce minimum experience requirements for nurses wishing to work with injectables, saying that they must have at least one year’s full-time experience as a nurse outside of non-surgical cosmetic procedures.
For years, nurses and dentists have operated in the billion-dollar cosmetic injectable industry without needing to complete any formal extra training or education before injecting patients with neurotoxins such as anti-wrinkle injections.
While many practitioners in the industry operate safely and comply with existing laws, regulators have flagged rising concerns about unqualified providers and inadequate oversight in some sectors.
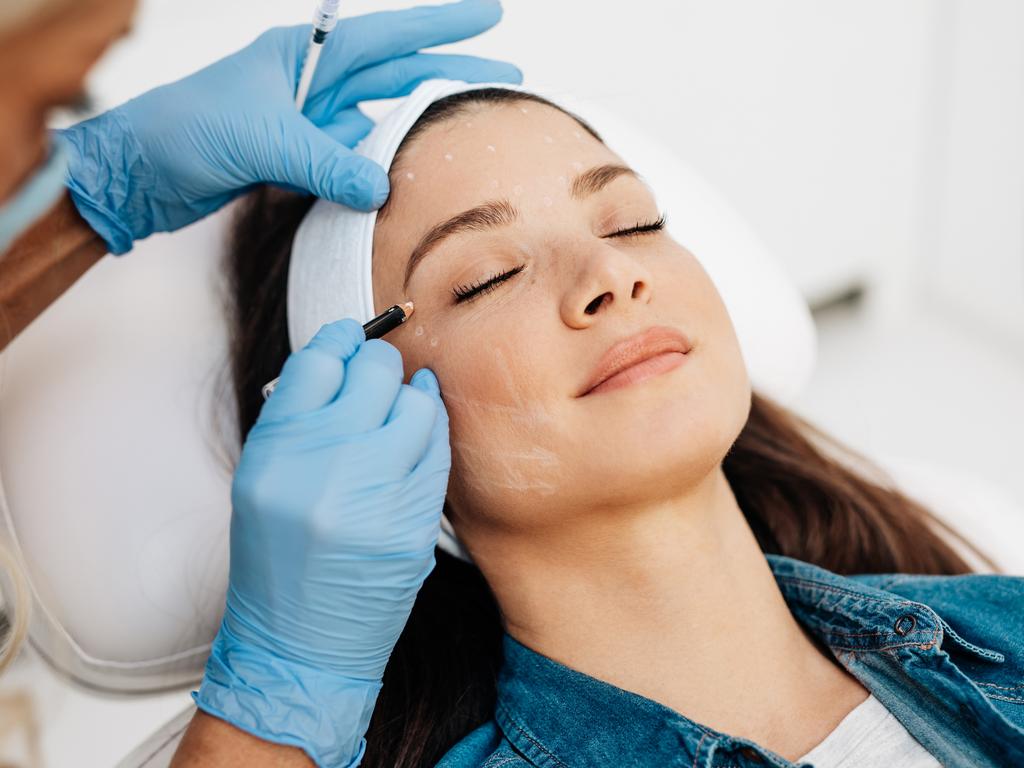
Crackdown on influencer testimonials and ads
The new changes also stipulate that advertisements for cosmetic injectables must include the details of the registered practitioner who will perform the procedures.
Targeted ads directed at minors will also be banned, along with testimonials from social media influencers, who will no longer be eligible for free or discounted cosmetic injectable treatments.
Justin Untersteiner, CEO of AHPRA, noted that not all cosmetic injectors would be pleased with the stricter regulations and he expected some would decide to leave the industry, according to ABC.
“There will be others who have to modify their business models and modify their practices to meet these guidelines,” he said.
“This is a growing industry and, I’ve got to say, what we do see in some cases is that there are people out there putting profit before patient safety.”
Nurses and dentists will need to implement the changes before September to ensure they are compliant with the new rules, which are in line with those already in place for doctors.
“(After that) we will be identifying those that are doing the wrong thing and we will take action where we need to,” Mr Untersteiner said.
Increase in ‘dodgy’ Botox
There has been a recent spike in cases involving unregistered practitioners and clinics using off-brand or outdated dermal fillers and anti-wrinkle injections on patients, resulting in multiple hospitalisations after patients fell ill with botulism.
In January, three people were treated in intensive care for suspected botulism, a potentially fatal illness, after reportedly receiving unregulated anti-wrinkle treatments at a home.
Dr Jeremy McAnulty, Executive Director of Health Protection at NSW Health, issued a health warning to those thinking about anti-wrinkle injections, highlighting the risks and side effects associated with unregulated cosmetic injections.
In March, a cosmetic clinic in Sydney received a health warning due to its “highly concerning” infection-control practices.
The NSW Health Care Complaints Commission (HCCC) advised clients to undergo testing for possible exposure to bloodborne viruses, including hepatitis B, hepatitis C, and HIV.
Speedy telehealth calls
The new federal guidelines mark the latest action by state and federal regulators, who have been ramping up their stance on cosmetic injectable providers.
Many injectable businesses across the country are operated by nurses who administer the injections, often stock prescription fillers and anti-wrinkle injections like anti-wrinkle injections on-site, and organise telehealth consultations for their patients so doctors can remotely prescribe the products.
A report by The Age in March revealed that these telehealth consultations saw doctors spend as little as 52 seconds with patients before writing a script for anti-wrinkle injections and fillers.
While the new guidelines don’t specify how long doctors and nurses must spend talking with patients before prescribing injectables, they do state that practitioners must conduct detailed evaluations, including assessing the patient to ensure expectations are realistic.
They should also discuss alternative options with patients and complete a lengthy checklist to confirm that patients have provided proper consent.
Peak body for cosmetic plastic surgery in Australia responds
Dr Lily Vrtik, a specialist plastic surgeon and President of the Australasian Society of Aesthetic Plastic Surgeons (ASAPS), welcomes the change but argues that more needs to be done.
“Non-surgical cosmetic procedures are growing rapidly in popularity, yet regulation and clinical standards have not been keeping pace,” Dr Vrtik said.
While ASAPS “welcomes the call by AHPRA for the requirement of appropriate education, training, experience, and ongoing education,” they need to crack down further on Continuing Professional Development (CPD) requirements.
“CPD is a condition of health professional registration,” she explained. “Practitioners who perform cosmetic injectable procedures should undertake regular, evidence-based training updates that are specific to cosmetic medicine and that include a strong emphasis on ethical practice.
“These new guidelines now stipulate that CPD should be in the area of their practice (i.e. cosmetic medicine), but alarmingly, there is no minimum quality standard for the CPD educational activity and no plan for enforcement.”
Originally published as Health regulator crackdowns on cosmetic injectables industry









