Off and running: EMVision begins pivotal trial of emu brain scanner to diagnose stroke
EMVision Medical Devices starts pivotal validation trial for its first commercial device the emu bedside brain scanner to diagnose stroke.

Stockhead
Don't miss out on the headlines from Stockhead. Followed categories will be added to My News.
EMVision starts pivotal validation trial for emu bedside brain scanner to diagnose stroke
The first Australian site is the Royal Melbourne Hospital
The first US site is the University of Texas Health Science Center at Houston (UTHealth) Medical School and Memorial Hermann-Texas Medical Center (TMC)
Special Reports: EMVision Medical Devices has kicked off a pivotal validation trial for its first commercial device – the emu bedside brain scanner to diagnose stroke.
EMVision Medical Devices (ASX:EMV) has announced Australian ethics approval has been granted and The Royal Melbourne Hospital (RBH) greenlit to start as the first Australian hospital after a successful site initiation visit.
EMVision said operator training was in progress at the RMH, which is considered a world-class comprehensive stroke centre, and home to the Melbourne Brain Centre – the largest brain research collaboration in the Southern Hemisphere.
The company said it was proud to continue its collaboration with the RMH team, who were instrumental to the success of the prior large, multi-centre pre-validation EMView study.
Device on way to first US site
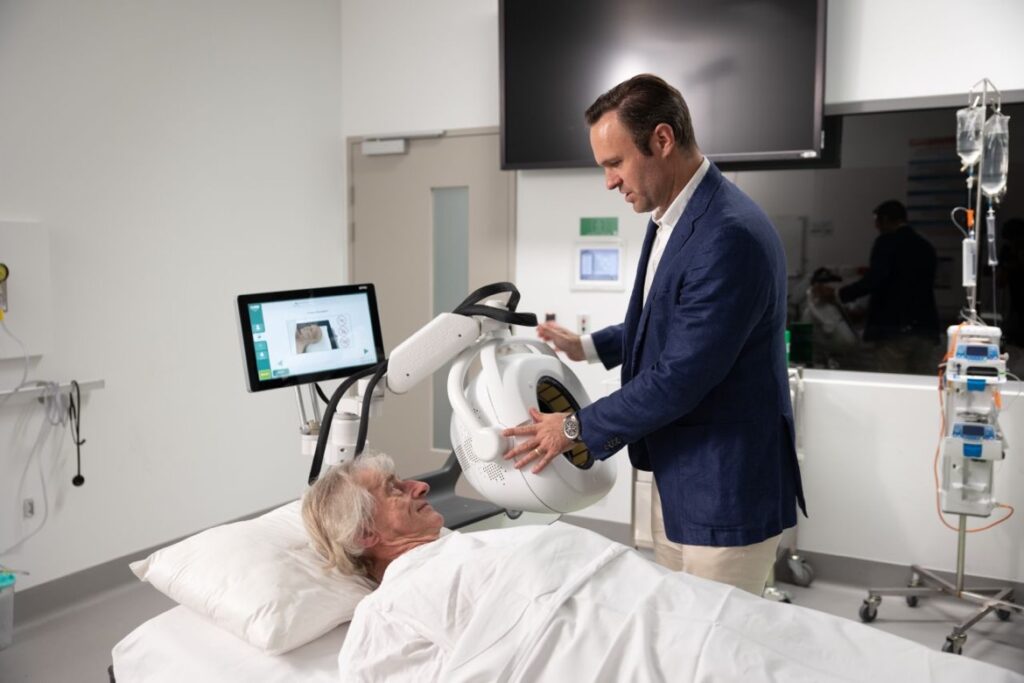
EMVision said an emu device had been shipped to the first US trial site, University of Texas Health Science Center at Houston (UTHealth) Medical School and Memorial Hermann-Texas Medical Center (TMC).
UTHealth has a long tradition of stroke care innovation, having pioneered the use of tissue-type plasminogen activator (tPA), a life-saving treatment for acute ischaemic stroke, along with mobile stroke units to expedite its delivery.
EMVision said a site initiation visit to UTHealth was planned for the coming weeks. Additional pivotal trial sites in the US and Australia are set to be named and activated shortly.
Rapid detection of strokes to minimise brain damage
As well as sharing a name with a flightless native Australian bird, emu refers to electromagnetic unit and is the most advanced of the company’s two trademarked products designed to rapidly diagnose stroke.
The company’s second device First Responder is a lighter version of emu and recently undertook initial proof-of-concept testing in collaboration with the Royal Flying Doctor Service and the Australian Stroke Alliance (ASA).
The brain scanners are non-invasive devices that use ultra-high frequency radiofrequency (RF) scanning technology, combined with advanced AI-based algorithms, to assist in point-of-care (POC) stroke diagnosis.
Strokes are best treated in the first hour – the so-called Golden Hour – post event.
In the company’s words: “Rapid triage, transfer and treatment decisions are critical to minimise brain damage, disability and death.”
A series of successful clinical studies have already been undertaken on emu, ranging from healthy human volunteer studies, through to a proof-of-concept study and the EMView study of patients experiencing acute suspected stroke.
Designed to support FDA de novo clearance
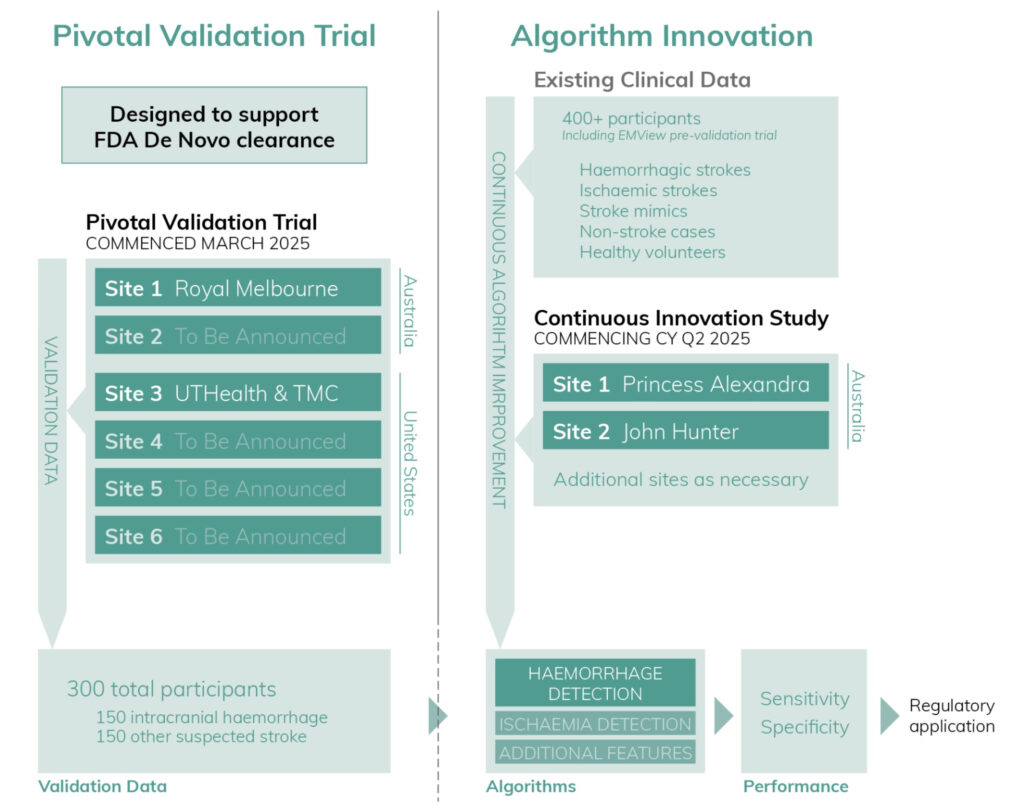
The trial is designed to support US Food and Drug Administration (FDA) de novo (new device) clearance for emu.
If granted clearance emu is anticipated to become the predicate device for First Responder allowing an expedited 510(k) FDA pathway for the pre-hospital market.
The emu pivotal trial has an estimated enrolment period of 6-12 months, followed by analysis and reporting of the data with the primary objective demonstration of haemorrhage detection sensitivity and specificity of >80%.
EMVision said determining the presence of haemorrhage is critical to selecting the treatment pathway, with the alternative ischaemic stroke diagnosis requiring a divergent treatment approach.
The company said 300 suspected stroke participants would be enrolled across four sites in the US and two sites in Australia.
Data will be collected in a way that allows sequential validation of additional features, such as ischaemia detection, without undertaking an additional full validation trial.
To ensure scientific validity, EMVision is blinded to certain study data and results will be analysed at the conclusion of enrolment.
Continuous innovation study to progress device features
In parallel, EMVision will run a continuous innovation study where additional patients will be scanned at multiple sites in Australia, including the Princess Alexandra Hospital in Brisbane and John Hunter Hospital in Newcastle.
The study data will be used to progress development of additional device features and expand the training library for EMVision’s diagnostic AI algorithms.
EMVision observed meaningful performance increases in the sensitivity/specificity of its diagnostic AI algorithms during the EMView study when additional training data was used.
Source: EMVision
‘Eager to see diagnostic power of the emu’
Neurologist and ASA co-chair Professor Stephen Davis AO said the organisation was pleased to see trials of emu in a hospital setting.
“We are eager to see the diagnostic power of the emu as its performance will also help inform the likely success of the First Responder device – a portable brain imaging tool that the Stroke Alliance aims to take on the road and in the air with emergency paramedics attending code strokes,” he said.
“This trial will support our quest to provide urgent onsite brain imaging – a critical first step in the stroke-treatment pathway.”
EMVision CEO Scott Kirkland said the company was excited to start the pivotal trial for emu to support market entry and looked forward to activating additional sites.
“This important milestone marks the culmination of many years of hard work and dedication from our team and our clinical collaborators, in pursuit of the development and validation of world-first neurodiagnostic technology that has the potential to significantly reduce the global burden of stroke,” he said.
This article was developed in collaboration with EMVision, a Stockhead advertiser at the time of publishing.
This article does not constitute financial product advice. You should consider obtaining independent advice before making any financial decisions.
Originally published as Off and running: EMVision begins pivotal trial of emu brain scanner to diagnose stroke