High-stakes race to vaccinate world against Covid-19 intensifies
Governments are scrambling to secure the small number of doses from Western companies as Russia and China race to fill the gap.
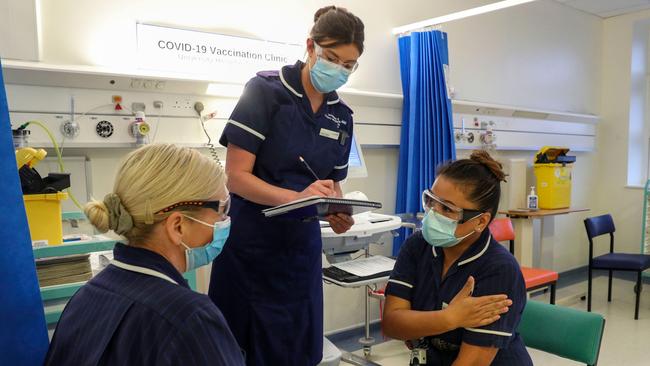
The race to vaccinate the world’s population against COVID-19 is shifting into a high-stakes contest, as governments scramble to secure the small number of doses becoming available from Western pharmaceutical companies and Russia and China race to fill the gap.
European and US regulators are expected to decide within weeks on whether to follow the UK in authorising a vaccine.
The result is a fragmentary global vaccination drive likely to proceed at vastly different speeds.
For most people around the world, a vaccine will remain a distant hope for several months, and it could be years before the poorest countries cover their people.
Consequently, governments might need to deploy alternatives such as widespread testing and smarter social-distancing restrictions in order to contain the virus’s spread, global public health experts said.

“When we talk about the global vaccine race, there are still a lot more questions than answers,” said Syra Madad, a senior director at New York City’s Health and Hospitals Corporation. “I don’t think it’s going to be enough to get us out of the pandemic … if the question is, is a vaccine an exit strategy? It’s part of an exit strategy.”

Europe and America have struck dozens of different, competing bilateral contracts with drug makers, but initial supplies will be limited, forcing hard choices through at least the first quarter of next year.
This past week, the UK’s authorisation of the West’s first vaccine offered hope that other leading Western candidates could soon cross the finish line. But the front-running candidates use novel viral RNA technology that is hard to scale up. Whether and how soon the US and EU can inoculate the majority of their populations will partly depend on whether other vaccines — which use more traditional platforms — prove safe and effective.
Poorer and less powerful states are meanwhile largely cut out, dependent on deals cut in Moscow, Beijing or by a network of World Health Organisation-backed public health institutions.

Russia and China have signed bilateral deals in the Middle East, Latin America and elsewhere to distribute their vaccines, but those shots still remain clinically unproven.
Chinese firms have yet to produce evidence from trials to convince sceptical regulators that their shots work, while Russia authorised its vaccine before trials were completed, leaving immunologists mistrustful of its safety and efficacy.
Moreover, neither is able to satisfy the gaping global demand: Russia has struggled to manufacture its Sputnik V vaccine at scale. China intends to produce more than one billion doses annually, and hasn’t said how many it will export.
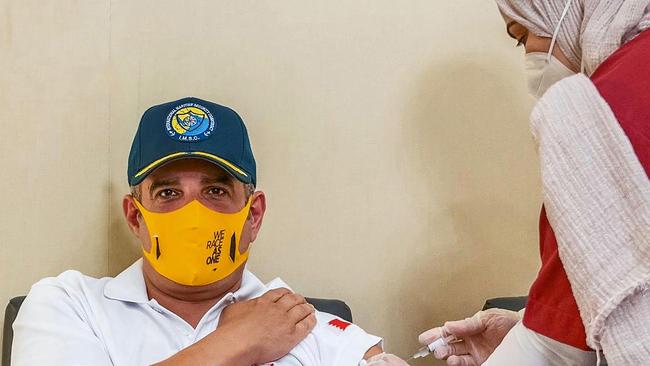
European and US regulators are expected to reach a decision within weeks on whether to follow the UK in authorising a shot by Pfizer Inc and Germany’s BioNTech. But that alone brings small relief.
The EU has only pre-ordered enough supply of the Pfizer-BioNTech shot to inoculate a third of the bloc’s 450 million people at most. The US has pre-purchased enough to vaccinate 50 million Americans.
It will be many months before those quantities are produced, as Pfizer has struggled to secure the raw materials and new technology it needs to produce even half of the 100 million doses it hoped to have ready worldwide by the end of this year. The UK, which expected to get up to 10 million doses this year, now anticipates receiving about half in that timeframe.

The next likely candidate, Moderna Inc, only has 160 million doses lined up for the EU and will make 20 million doses of its vaccine available for the US this month. An additional 85 million to 100 million doses are expected to be available for the US during the first quarter of 2021.
Moderna’s vaccine, as well as Pfizer’s, requires two doses. The vaccine uses similar mRNA technology as Pfizer’s, and the manufacturing process is challenging. It involves injecting tiny strands of viral DNA into a microscopic nanoparticle of fat at a stable temperature, without allowing oxygen to seep into the materials — on an industrial scale.
That leaves Europe and America dependent on less cutting-edge vaccines that are far easier to produce, but whose future is cloudier. The most important of those is a two-dose shot by AstraZeneca and Oxford University. The EU has purchased 400 million doses and the US has bought 500 million.

But there are questions about the shot’s effectiveness, after the company said thousands of its test subjects were accidentally given a lower dose than intended during trials. Other vaccines, from Sanofi SA, Johnson & Johnson, and Novavax Inc, are months away from approval in the EU, and there is no guarantee they will prove effective.
Beyond Europe and America, the world’s poorest countries are counting on the Geneva-based public health institution GAVI, which has raised $US2 billion ($2.7bn) in a bid to vaccinate as much as 20 per cent of the population of about 92 developing countries.
But GAVI’s WHO-backed vaccine drive is also heavily dependent on AstraZeneca’s shot, of which it has pre-ordered 300 million doses.
Even if that vaccine proved effective, it was likely to be in short supply for the near future, GAVI chief executive Seth Berkley said.
“When we look at the end game of this pandemic, vaccines are likely to be a crucial part of the puzzle, but they also need to be acting alongside improved testing, widely available treatments and improved health systems,” he said.
“It is not good in a pandemic to allow the virus to be mutating and continuing to spread uncontrolled.”
Meanwhile, China is looking to fill that gap with three leading candidates. Tens of thousands of government employees and soldiers in the United Arab Emirates have received one Chinese vaccine, and more than one million doses of a vaccine developed by China’s biopharmaceutical company Sinovac have arrived in Brazil.
At home, Chinese authorities have inoculated nearly one million Chinese people with a COVID-19 vaccine from state drugmaker Sinopharm, which has yet to provide any clinical evidence of the shot’s efficacy.
Countries such as Indonesia and Turkey are beginning to order Chinese vaccines while holding off on using them, because China has yet to release efficacy data.
“We need to adhere to scientific and medical standards, with vaccines passing all three clinical trials,” said Wiku Adisasmito, head of Indonesia’s COVID-19 response task force. All told, Turkey has pre-ordered 50 million doses.
Meanwhile, African countries are trying to understand not only which vaccine candidates are available to them, but the feasibility of delivery. Ethiopian Airlines said on Wednesday it was setting up the airfreight facilities to help Cainiao, the logistics arm of Alibaba, distribute Chinese vaccines in Africa.
“We’re talking about a continent where the supply chain for basic drugs isn’t even developed,” said W. Gyude Moore, a senior policy fellow at the Centre for Global Development, a nonpartisan think tank in Washington, DC. He said that Africa probably wouldn’t be able to start using Chinese vaccines until late February.
In Russia, large-scale vaccinations against COVID-19 began in Moscow on Saturday.
Some countries — Mexico, India and Egypt — are counting on receiving tens of millions of doses of that shot, but Russia has struggled to produce even two million of the 30 million doses it hoped to manufacture for its own needs by the end of this year.

Attempts to ramp up production have stumbled as scientists have struggled with fine-tuning various technical processes and have encountered issues with the equipment.
“We’re all focused on the Pfizer vaccine, but rapidly we will start to look at a whole range of different vaccines,” Professor of Virology Deenan Pillay at University College London said.
“From a public health perspective, this becomes one more thing to reduce deaths, but it is essential that we don’t fall into complacency.”
Dow Jones Newswires




To join the conversation, please log in. Don't have an account? Register
Join the conversation, you are commenting as Logout