Could ultrasound be used to treat Alzheimer’s?
A Brisbane scientist is convinced it can – and clinical trials on people are about to begin.

You might think there was a Eureka moment for Jürgen Götz, a jolt of inspiration that hit the Brisbane scientist like a thunderclap and opened his mind to all the dazzling possibilities his research now presents. But that’s not how science generally works, and it’s certainly not how his target, Alzheimer’s disease, takes hold of the human brain.
Götz’s progress was slow, painstaking, incremental. It took nearly a decade to test his idea on mice and sheep and to build a machine to implement it – something that looks like a computer trolley you might wheel around the office. And if it is safe and helps people as it’s supposed to, this might turn out to be one very big deal indeed. A ray of hope in the obsidian gloom of dementia.
Let’s face it: it’s what many of us fear most about growing old. To lose memory, cognition, ourselves, in a long descent into oblivion. As the nation’s population ages, more people will come down with dementia-related illness – especially Alzheimer’s, which accounts for more than 70 per cent of cases. Small wonder the field is such a hotbed of scientific endeavour. The glittering prize, of course, is a drug to arrest the onset of symptoms or, better yet, reverse them.
Over the years, there have been more false starts than German-born, Swiss-trained Götz, a professor of dementia research, can count. The latest sensation, a drug called aducanumab, featured in these pages in July after receiving fast-tracked approval in the US. It attacks the toxic protein amyloid, which accumulates as plaque in the brain and is intimately associated with Alzheimer’s disease. Although the drug’s performance at the physiological level is striking – it can shrink decades’ worth of accumulated amyloid deposits to near-normal levels in 12 months – the effect on patient outcomes has proved to be less compelling. At best, cognitive decline was slowed among some early-stage sufferers in the clinical trials; their neurodegeneration would continue.
That’s not the only catch. At $72,000 a year, aducanumab would be prohibitively expensive to subsidise were it to be made available in Australia. (The Therapeutic Goods Administration is tipped to hand down a decision on its registration as a medicine in the new year.) The row in the US over the expedited approval process compounded scepticism about the drug; three members of an expert advisory committee to the US Food and Drug Administration quit, complaining of “excessively optimistic early messaging”.
Then there’s tau, the less-known cousin of amyloid. As plaque levels build up in the brain, these spaghetti-like tangles erupt within nerve cells, eventually overwhelming them. Götz believes tau is just as destructive to brain function, maybe more so, yet the research focus remains locked on amyloid. As we will see, the medical science in this space is deeply contested.
But what if there were a cheap, well-understood and accessible therapy out there waiting to be repurposed? And what if it could break down both amyloid and tau, while also breaking through the imposing seal of the blood-brain barrier to deliver medicines that would otherwise be blocked and dramatically improve their uptake to boot? Enter ultrasound, technology so humdrum that it takes a bit to get the head around why Götz and his team are convinced it can help Alzheimer’s patients. Yet the early results are in: the sonic energy system most Australians would associate with the sticky caress of an obstetrician’s wand works such a treat in the animal models that it has, in some cases, restored cognitive function destroyed by the disease. Now it’s time to try it on people.

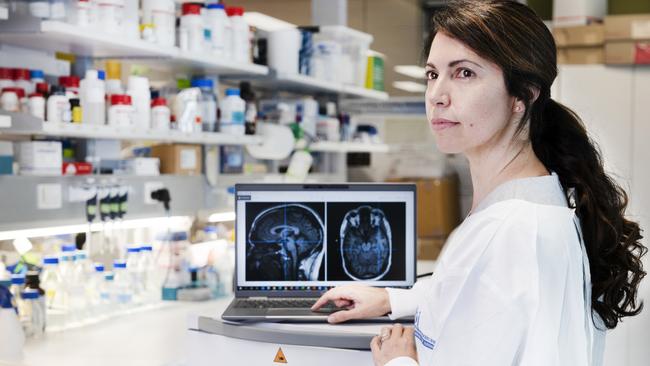
We are sitting in a small, ordered office on theground floor of the Queensland Brain Institute at the University of Queensland (UQ) while Götz explains what all the fuss is about. He’s a trim, neatly groomed man who looks at least a decade younger than his 60 years and speaks in an accented, rat-a-tat staccato. Senior research assistant Wendy Lee has fired up a prototype of the machine engineered to bombard the human brain with ultrasound. The magic is in the programming, perfected through trial and error with mice to prove the concept, and then sheep to fine-tune the delivery mechanism (at 7mm thick, a sheep’s skull is close to that of the average person). The frequency is set at 286kHz, meaning the sonic waves cycle at 286,000 times a second in intricately calibrated pulses. Assuming ethics sign-off, 12 volunteers with early to mid-stage Alzheimer’s will be dosed four times at fortnightly intervals.
The aim is to demonstrate the treatment does no harm, the first and overriding concern in any clinical trial. Götz is confident the strong results in the mouse studies will be replicated: his 2015 paper caused an international stir when it appeared on the cover of the prestigious journal Science Translational Medicine, describing how ultrasound had reduced amyloid build-up by 60 per cent in genetically engineered lab mice. Follow-up testing showed the animals had regained lost memory function, prompting him to declare to the media: “The word ‘breakthrough’ is often misused. But in this case, I think this really does fundamentally change our understanding of how to treat this disease.”
Think of what a dental descaling device does to shake loose oral plaque from a tooth. The theory is that low-intensity ultrasound exerts a mechanical force that does that and more in the brain: applied correctly, it can “melt” targeted tissue, Götz says. By using pulsing, the pressure wave stimulates a range of cellular activity targeting both types of rogue protein. Activated microglial cells, so-called “garbage” eaters, break up and digest clumps of amyloid plaque. In a separate, autophagic response, tau concentrations are also reduced, though not as markedly. “The good thing about ultrasound is that it’s like aspirin… it performs multiple, different jobs,” Götz enthuses.
When paired with microbubbles administered by an intravenous injection to the arm – an established method to enhance medical scans – the blood-brain barrier is levered open. This is no mean feat. The endothelial cells lining blood vessels in the brain are wedged tight to prevent pathogens or impurities being absorbed, walling off the body’s most delicate organ. The downside is that only minute doses of medicine can get through.
After passing through the endothelial membrane, the microbubbles expand to the thrum of ultrasound. A passive cavitation detector allows the operator to visualise the blood-brain barrier parting, a novel feature of the technology. The effect is temporary and the protective lining snaps back within 24 hours. But that’s long enough to supercharge the effect of a treatment such as aducanumab. “All the evidence indicates that what we are seeing in animals would also work in humans,” Götz says. The scale might be different – a mouse brain is the size of your fingernail – but the physiology matches up.
If only it were that easy. Dealing with Alzheimer’s disease is like wrestling jelly. Since the German psychiatrist Alois Alzheimer cut into the brain of a deceased dementia patient in 1906 and identified the cottony deposits now known to be amyloid plaque, just about everything that can be challenged has been called out, down to whether the woman concerned had another form of dementia. Scientists still can’t agree if amyloid and tau cause the problem or are by-products of it, though the consensus is they’re integral to what happens to Alzheimer’s patients. Nor can they explain why some people can carry a heavy amyloid burden into old age without evident ill-effect.
“We don’t really have a full understanding of what’s driving this process,” says Peter Nestor, the UQ professor of neuroscience who will be the linkman between Götz’s team and the specialist company that will independently manage the Phase 1 clinical trial. “When you look at the brain of someone who has had Alzheimer’s disease under the microscope, there are these characteristic pathological changes, amyloid and tau, and the aim of a lot of research is to try to remove these abnormalities in the hope it will stop the brain from degenerating,” Nestor says. “But given that our knowledge of all these dementia-related diseases is incomplete, that might be too simplistic. There could be some upstream event we don’t understand that is giving rise to the accumulation of these abnormal proteins and also causing cognitive loss.”
Alzheimer’s is a graveyard for big pharma. The field is littered with candidate drugs that looked the goods in animal studies but failed when tested on human subjects. Geriatrician Michael Woodward, a medical adviser to Dementia Australia and head of aged and residential care services at Austin Health in Melbourne, agrees the ultrasound results look promising. So did the drug being developed by California-based biotech company Cortexyme to test a new hypothesis linking Alzheimer’s to inflammation caused by gum disease – until it too fell over deep into a combined Phase 2-3 clinical trial in October. For all its limitations, Biogen’s aducanumab is one of only a handful of medications to be licensed to treat Alzheimer’s, and the first to show potential to arrest the disease’s progress.
Woodward points to another high-profile bust, semagacestat, part of a class of experimental drugs that went after the precursor protein for amyloid. “They looked very good in mouse models,” he says. “They did the job in mice that were genetically bred to over-express amyloid… they basically stopped this happening and they stopped the mice from developing the sort of behavioural and cognitive changes that are the mouse equivalent of Alzheimer’s. But they didn’t work in people. In fact, they caused it to worsen in people.”
A missing piece of the jigsaw is that science is still a long way from unwinding the mysteries of the human brain. “It’s basically a fully functioning computer made of fat,” Woodward continues. “Now, you show me someone who can go even close to saying how that all works and I’ll be the first to shake their hand… there are just so many things going on: 15 neurotransmitters, 10 major cell types, all doing different things in different situations at different times of our lives.”
Consider memory loss, the telltale symptom of Alzheimer’s. Scientists know where memories are stored in the brain (the hippocampus, the first area to be affected by Alzheimer’s, banks long-term memory), but take another step back. What even is a memory? “To be absolutely honest, no one understands how memories form,” Götz says. “And we don’t really know how memories are coded in the brain. We are kind of in the dark.”
But back to that light bulb moment, or lack thereof. Götz can’t recall exactly when he decided to apply ultrasound to Alzheimer’s. The general idea came to him at a scientific conference soon after he was recruited to head the Clem Jones Centre for Ageing Dementia Research, part of the Queensland Brain Institute. He chanced upon a poster for ultrasound and it just twigged: maybe he could apply it to his Alzheimer’s work. “It’s not a particularly exciting story,” he says dryly.
By 2012, research at UQ had advanced far enough for his team – today numbering 35, 10 of whom are full-time on the ultrasound project – to go for a grant from the National Health and Medical Research Council. The application was rejected. They received a second knockback in 2015 despite the splash made by the cover piece in Science Translational Medicine. The private feedback from a member of the NHMRC panel: “It was not innovative enough, it was not exciting enough, it was not tested enough,” Götz recalls. But the team pressed on with a combination of university and philanthropic backing. More than $30 million was raised to get the technology to where it could be tested on people.
Early on, Götz had brought in a commercial specialist, Rachel de las Heras, a molecular biologist who’d spent years in private biotech bringing new medical devices to market. This was a departure from how the university sector traditionally operates: scientists work the science and worry about the business side later. To date the project has been kept in-house instead of being spun off into a startup company or licensed to an industry player, a further point of difference.
De las Heras, 46, says devices are a harder sell because the big dollars in medical R & D still go to drug development. “The perception is a medical device won’t return the same quantum… but that ignores what’s happened in the Alzheimer’s space. For the most part, drugs haven’t worked,” she says. “There’s been over 200 trials that have failed. So at what point do you keep investing in a drug-only holy grail and start thinking outside of that?”
Götz says the choice needn’t be binary. As he sees it, ultrasound therapy would go hand-in-hand with emergent drug treatments. “It’s not the device on the one hand or the drug on the other… it’s about bringing them together,” he says. “Ultimately, one might combine ultrasound with aducanumab or some other monoclonal antibody treatment. We have shown in the animal experimentation the combination is really powerful.”
He is all too aware of the personal dimension, the hope invested in research when the odds are stacked mightily against people struggling with Alzheimer’s. He’s taken the anguished calls from patients or their loved ones begging for access to the program, anything that might help. “They can’t stop crying. It’s tragic,” he says quietly.
No doubt this story will invoke a fresh flurry of pleas. Götz accepts he has a responsibility to manage the expectations when there’s no silver bullet, nothing approaching a cure in sight. But the longer-term outlook could be brightening. Woodward says aducanumab represents an important step in the right direction, if not the leap forward it was hyped to be. Cholesterol-lowering statin drugs looked to be a lost cause at one stage of their development, but the researchers persevered and untold millions are living longer as a result. “Progress doesn’t always happen in a straight line,” Woodward says.
Götz, picking up on this, says Alzheimer’s will probably always be with us. Like cancer, it might be nature’s way of telling haughty humans that time is up. After age 65, the risk of developing Alzheimer’s doubles every five years. So Götz believes the aim should be to delay onset and contain the symptoms for as long as possible. He’s encouraged by advances to identify biomarkers of disease earlier. If ultrasound succeeds in the upcoming clinical trials – that big “if” again – it could be part of an intervention to arrest amyloid accretion before the plaque can do damage.
“It is a disease modifying strategy,” he says. “The age of onset is pushed out. You don’t fix the underlying problem at its root, but you remove whatever has built up. It’s like removing rust… you get rid of the stain but the metal continues to rust; you haven’t removed the problem but it doesn’t matter.”
Could people who have lost memory have function restored, as happened with his lab mice? Well, that depends, Götz answers warily. “I think with improved biomarkers we can diagnose earlier and treat earlier and it should be possible to… contain it provided amyloid and tau are driving the disease, as I believe. But that is quite a lot of steps away from where we are.”
The timing of the safety trial is dependent on the overseeing ethics committee approving the terms and structure of the study, and Götz says that can’t be taken for granted. If all goes smoothly, though, recruitment should start in the new year with candidates passing rigorous inclusion and exclusion criteria. Before anyone reaches for the phone to vie for one of the 12 spots, they should know Götz can have nothing to do with the exercise: the researchers must be at arm’s length to preserve the integrity of the process. (Details of the company conducting the trial won’t be made public, either.) The true test will come later, after microbubbles are introduced to open the blood-brain barrier, followed by a monoclonal antibody agent (the lab has developed its own and used the drugs on mice) to exploit the breach, assuming the program gets that far. Only then will the treatment’s efficacy be benchmarked.
Götz believes there may be other applications. Ultrasound has been approved in the US to treat essential tremor, a neurological disorder that causes shaking, and is being explored for use against brain cancer and prostate tumours in men, as well as non-malignant breast and uterine fibroids in women. Ultrasound has “tremendous possibilities” beyond diagnostic imaging, he says. But let’s not get ahead of ourselves. The challenge of Alzheimer’s is daunting enough.
For Raymond, a 79-year-old retired academic who was diagnosed with Alzheimer’s two years ago, it’s a rollercoaster of good days and bad days at home in Brisbane with his wife Kate, 64. She says they are learning to live “in the moment” with the disease. Sometimes, she has to tell herself he’s still the same kind and loving man she married 47 years ago. “When things are a struggle, you realise you just have to go with it. Our life can’t go back to what it was.” Raymond has put his hand up for the upcoming clinical trial. He knows it is probably too late for any new treatment to help him. But that’s not the point. “This is about everyone’s future,” he says.

