Cancer’s cruel lottery
Not all sufferers are created equal when it comes to receiving help despite the many advances that are being made.

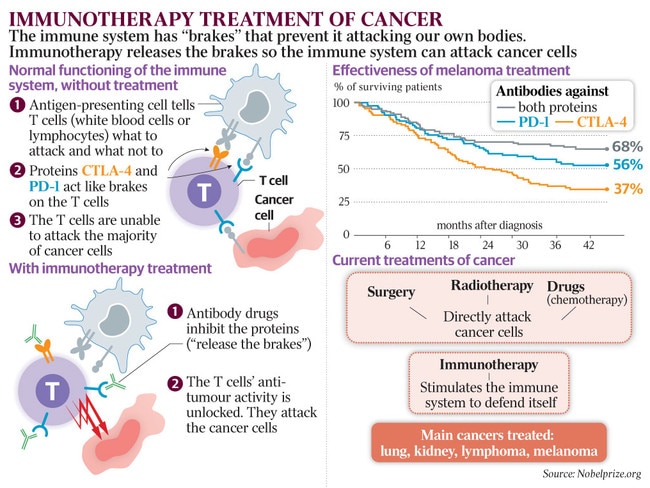
The good news for cancer patients is that at long last the promise of personalised genomic medicine is beginning to feed into results. Lives are not only being prolonged, they are being saved.
If only that were the whole story. In the real world there’s always a catch. And with this technology, as with most advances that happen in life, there’s a lag between what scientists know they can do to help sick people in the direst of circumstances and what the system allows.
That means the odds in the grim, pitiless lottery of cancer can be stacked higher against the unlucky individual: have a lump appear in a breast or lung and you will benefit from decades of intensive worldwide research conducted at untold expense, but contract one of the so-called rare cancers that collectively account for about a third of all cases in Australia and the help is far more limited; live on the wrong side of the river in Brisbane and you will be ineligible for free genomic panel testing that opens up a host of cutting-edge options for treatment.
What’s more, Dr Google lets patients know exactly what they’re missing out on. They can read about the new drugs that are causing a stir overseas, yet are not accessible, or prohibitively priced, here. In lung cancer, where mainstream treatments are increasingly based on gene therapy, only three or four dozen known mutational targets are screened in routine testing. If the patient pays to have a panel sequence done — at a cost of up to $6000 — they still have to find a doctor willing and able to act on the information.
The frustration among cancer patients is palpable. “It’s an equity issue, absolutely,” says 40-year-old Canberra teacher Sarah McGoram, who has spent half her life hopping from one treatment or clinical trial to another to stay a step ahead of incurable GIST (gastrointestinal stromal tumour), a cancer so rare fewer than 15 Australians have it at any given time.
“I might be on a drug that I have to fight to access on compassionate grounds or on a trial, or face paying thousands and thousands of dollars for. But the guy standing next to me is getting the same drug virtually free on the PBS (the government’s Pharmaceutical Benefits Scheme) because his cancer is in a different part of his body to mine and because of that it’s scheduled (on the PBS). I know the argument about resources but the inequity that can happen across diseases, rare versus common, is staggering when we are all Aussies, all paying our taxes.”
The need to do things differently, to be smarter and more targeted in directing those precious taxpayer dollars, occupies medical scientist and clinician Matt Brown, director of genomics at Queensland University of Technology. A protege of former Australian of the Year Ian Frazer, inventor of the world’s first cancer vaccine against cervical tumours in women, he runs a genomic testing program through the Australian Translational Genomic Centre in Brisbane, a partnership between the Queensland government, QUT and state agency Pathology Queensland, operating out of Frazer’s Translational Research Institute on the campus of Princess Alexandra Hospital.
Yes, it’s quite a handle. But it’s important to understand the linkages because this is where cancer treatment is headed. And make no mistake, you have a direct personal stake in this: according to the Cancer Council, one in two Australians will contract the disease in their lifetime. The idea is to put researchers side by side with working doctors to drive innovation. In the ATGC program, that means front-loading genomic screening.
Rather than testing for a specific genetic mutation, say epidermal growth factor receptor in non-small cell lung cancer, which has a targeted drug therapy attached, the net is spread across a panel of 500 biomarkers.
A baseline sample of the patient’s DNA is then compared with the profile of their cancer, giving clinicians a more complete picture of the disease and, hopefully, new pathways to attack it.

Brown’s team has zeroed in on lung cancer and blood cancers, though most forms of the disease were represented in the 500 patients they screened last year in the opening phase of the program. As reported in the news pages, the results have been eye-catching. Fully 40 per cent of lung cancer patients and 60 per cent of those with blood cancers such as leukaemia and lymphoma had “clinically actionable” mutations that would not have been picked up by standard pathology tests; given that many of these people were out of conventional options, this opened the door to new, life-sustaining treatments.
In the case of blood cancer it can also guide the crucial clinical decision of when to commit to bone marrow transplantation, an expensive and invasive procedure with potentially lethal side effects. Data from the panel testing is run through an algorithm to predict with “moderate accuracy” a patient’s response to chemotherapy, Brown says. This suggests the frequency of bone marrow transplants could be cut by 25 per cent without affecting survival rates, a massive cost saving to the health system, given the treatment has a six-figure price tag, not to mention the physical benefit to patients.
“It’s not a crystal ball to predict how someone is going to do, but it gives you a reasonable level of confidence that they are going to do well with chemotherapy and, if so, they won’t need to go to transplant,” Brown explains. “It can really make a big difference to the quality and effectiveness of the treatment.”
The experiences of McGoram, who is enrolled in a national, federally funded genetic medicine program run by Sydney’s Garvan Institute of Medical Research, and 51-year-old Brisbane nurse Susie Krimmer are worth contrasting. Ten months ago, Krimmer was close to the end of the line with conventional treatment. Her metastatic breast cancer, seeded from an undetected tumour beneath the right armpit, had gone to her brain. A slight tunnelling of her eyesight in August 2017 turned out to be the giveaway symptom.
Initially, radiation treatment seemed to do the trick. Her doctor was confident the disease was in check; then, in April last year, the cancer was found to have spread to her liver and Krimmer was told she had two years at best to live. “I said, ‘No, no, you can’t be telling me that. This is my worst nightmare’,” she recalls. “On the way home … I wailed like a little cat.”
Krimmer ended up on Brown’s service through her own dogged perseverance, contrary to the well-meant advice of the senior oncologist she consulted, a family friend who insisted that genomic medicine was “the way of the future” and could not help her. Her own doctor’s eyes lit up when the sequencing results came back: a mutation had been detected that could be targeted by an immunosuppressant called everolimus, conventionally used in transplant medicine or to treat renal tumours.
The reversal of her fortunes has been nothing short of amazing. The four tumours in her brain are inactive, as are the two growths on her liver, which have shrunk. Krimmer is in thriving remission, looking forward to travel and to moving house, confident she has a future with her 15-year-old son, Luca. They are all too aware of how lucky she is — not only to be alive but that her address was in riverside Bulimba, in the catchment of the PA Hospital and Metro South Health. Had she lived on the other side of the river she would have been ineligible.
Brown argues that genomic testing needs to be ramped up in a systematic rollout, much like what’s happening in the case of Britain’s National Health Service under a drive to sequence the genomes of 100,000 people with rare diseases, plus their families, and patients with common cancers.
There are cost implications, of course. The tests now run to about $2000 each, though at the ATGC that includes the added expense of whole exome sequencing. Brown is in the process of formulating a formal economic assessment to government to widen the scheme.
“That’s obviously not a cheap thing to do, but on the other hand it does make a significant difference to the management of a large number of patients,” he says. “And we know that patients are missing treatments because of the lack of testing they should have.”
Even so, not everyone can be helped. McGoram was disappointed that her screening by the Garvan Institute failed to turn up a new target for gene therapy. She insists the testing was valuable as “future-proofing”, a nod to how quickly the science is evolving. Since the age of 18, when she was given only 12 months to live with GIST, she has been on a white-knuckled ride of surgery, new treatments and drug trials to keep ahead of the cancer as it adapted to whatever doctors threw at it.
For now, she’s being given compassionate access to the multi-kinase inhibitor regorafenib, a medicine that would otherwise cost more than $100,000 a year. “We are hopeful,” she says on behalf of husband Tom, 46, and their son, George, 12, who has just started high school in Canberra. “As the last 20 years have proven, there is always research that pops up.”
The head of Garvan’s cancer division, David Thomas, says its sequencing results are broadly consistent with Brown’s, uncovering useful biomarkers in about 45 per cent of rare cancer patients. But he’s deeply conscious of the responsibility of researchers not to give false hope to cancer patients — or to talk down existing treatments.
“What’s new about this technology where we are doing panels, or what I would call multi-gene tests, is rather than doing those things as a single test, we are doing them in one go,” he tells Inquirer.
“There are doubtless efficiencies to be had from that and there is future-proofing as well. But … in terms of the message to patients, because some people will quite understandably be desperate to interpret this in the most optimistic light or, significantly, they may see it as a cause for grievance, I would say that this is a field which is evolving and not yet laid down in law.
“It is important to understand that while it is promising, this is a field of active research rather than something which has clearly proven benefit. This represents an advance but it is not a qualitative advance; it’s a quantitative advance in the efficiency of testing.”