Therapeutic Goods Administration gives green light for early Pfizer vaccine
The nation’s medicines regulator has paved the way for an early release of Pfizer’s vaccine.

The nation’s medicines regulator has paved the way for an early release of Pfizer’s breakthrough COVID-19 vaccine by placing the drug on a priority path to approval, despite human trials not concluding until late this month.
The Therapeutic Goods Administration has already begun assessing the safety of the Pfizer coronavirus vaccine — known as BNT162b2 — by granting it a provisional determination reserved for urgently required drugs. Pfizer expects to have final results of human trials in three weeks, clearing the way for it to begin rolling out the vaccine, which has an efficacy rate of 90 per cent in interim data made public on Monday. Those results showed no serious safety concerns.
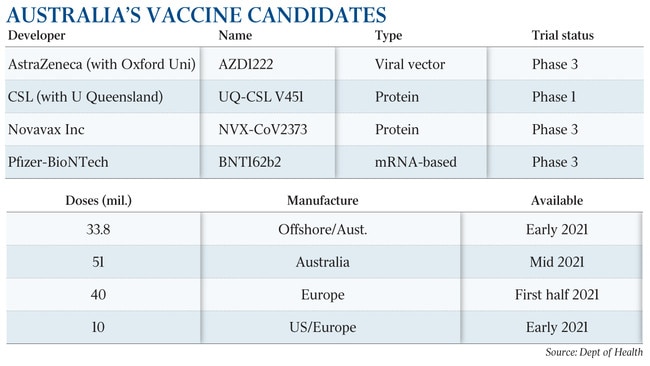
Australia will not be able to manufacture the vaccine, which uses new mRNA technology, with CSL — the only pharmaceutical company with a capacity for large-scale local manufacture — confirming it had no plans to make the vaccine.
The commonwealth last week struck a deal with Pfizer to import doses for five million people, although an inability to manufacture locally could slow supply,
The government estimates the doses will be approved in the early months of 2021, with Health Minister Greg Hunt confirming health and aged-care workers as well as the elderly and vulnerable would be vaccinated first.
The provisional determination means the TGA is already examining safety data and co-ordinating with overseas regulators to cut time to approval.
National Centre for Immunisation Research and Surveillance director Kristine Macartney said the status meant the TGA was “saying that they review everything about the context, they give the company the green light to come forward in this more streamlined, rapid rolling evaluation pathway”.
The TGA said issuing a provisional determination did not mean a vaccine would be necessarily approved but it fast-tracked the process. “The provisional pathway provides a formal and transparent mechanism for speeding up the registration of promising new medicines with preliminary clinical data,” it said in a recent statement.
Pfizer, which has developed the BNT162b2 vaccine with German partner BioNTech, released interim data from its phase-three trial of the vaccine on Monday, sending markets soaring. Pfizer chairman Albert Bourla said the data’s release was “a great day for science and a great day for humanity”.
“We are reaching this critical milestone in our vaccine development program at a time when the world needs it most, with infection rates setting new records, hospitals nearing overcapacity and economies struggling to reopen,” he said.
While there have been few new coronavirus cases in Australia — with no new local infections recorded on Tuesday — the virus continues to spread overseas including in the US, which had 130,553 new cases on November 9.
Scott Morrison said a vaccine would be made available “first and foremost on the basis that they are safe”.
“I don’t want to overstate it, but that is welcome news,” the Prime Minister said.
“(The Pfizer drug) is one of four vaccines that Australia is involved in and these results are very promising and I am optimistic and hopeful.”
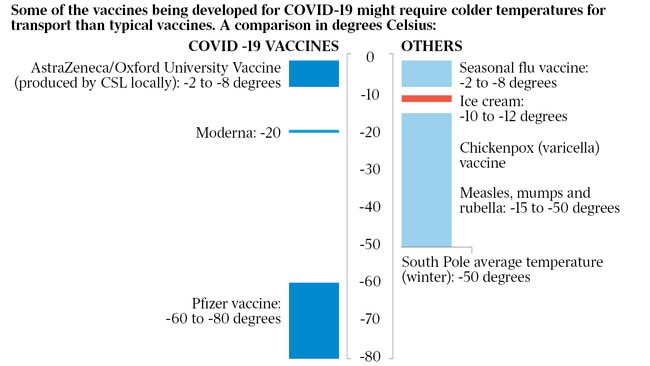
British health authorities have suggested the Pfizer vaccine could be manufactured by Christmas. Experts cautioned, however, that the interim efficacy data has not yet been peer-reviewed.
Pfizer-BioNTech has enrolled 43,538 participants in its phase-three trial of BNT162b2. The trial began on July 27 and so far, 38,955 people have received two doses of the vaccine.
During the trial so far, 94 people have contracted COVID-19, but only 10 per cent of those people received the vaccine rather than a placebo. Pfizer said it would continue the trial until there had been 164 confirmed cases of COVID-19 among participants.
It said it expected to be able to provide the US Food and Drug Administration with the required amount of safety data for emergency use authorisation of the vaccine by the third week of November.
Another mRNA vaccine — being developed by the US biotech Moderna — is also expected to deliver phase-three efficacy data in coming weeks.
Experts say it’s not a surprise that mRNA vaccines are emerging as the first to report efficacy data because mRNA vaccines are extremely quick to develop. They work by inducing the body’s cells to make the coronavirus’s spike protein, which then gets exported out of the cell, triggering the body to mount an immune response to the foreign protein.
Monash University is also working on an mRNA vaccine.
Pharmaceutical biologist Colin Pouton said the technology was the most promising development in vaccine development for decades but “to get 90 per cent efficacy is probably higher than anyone expected”. CSL has begun the manufacture of the University of Oxford-Astra Zeneca COVID-19 vaccine, and has committed to manufacture the University of Queensland’s vaccine should it prove effective in clinical trials.