Funding verdict looms on ‘future of cancer therapy’
The survival of hundreds of Australians living with a deadly type of blood cancer is in the hands of a government committee which will decide whether to fund a revolutionary therapy.

The chance of survival for hundreds of Australians living with a deadly type of blood cancer is in the hands of a government committee, which will decide whether to support public funding for a revolutionary therapy that could send their cancer into remission with a single infusion.
CAR T-cell therapy, a type of personalised immunotherapy that re-engineers a patient’s own immune cells to kill cancer cells, was approved earlier this year for multiple myeloma by the medical regulator for those who had failed conventional treatment and doctors have now launched an urgent bid for its public funding.
It’s the second time the Medical Services Advisory Committee has been asked to approve public funding of the new drug cilta-cel, sold as Carvykti, for multiple myeloma, with the lives of 300 patients who have failed conventional treatments hanging in the balance. The committee meets to consider the application on Wednesday.
“This therapy would be highly likely to significantly prolong these patients’ lives and in some cases, it could well be curative,” said Andrew Spencer, head of Malignant Haematology, Transplantation and Cellular Therapies Services and head of the Myeloma Research Group at Alfred Health in Melbourne. “And this is a disease which is considered, for intents and purposes, to be incurable with current therapy.”
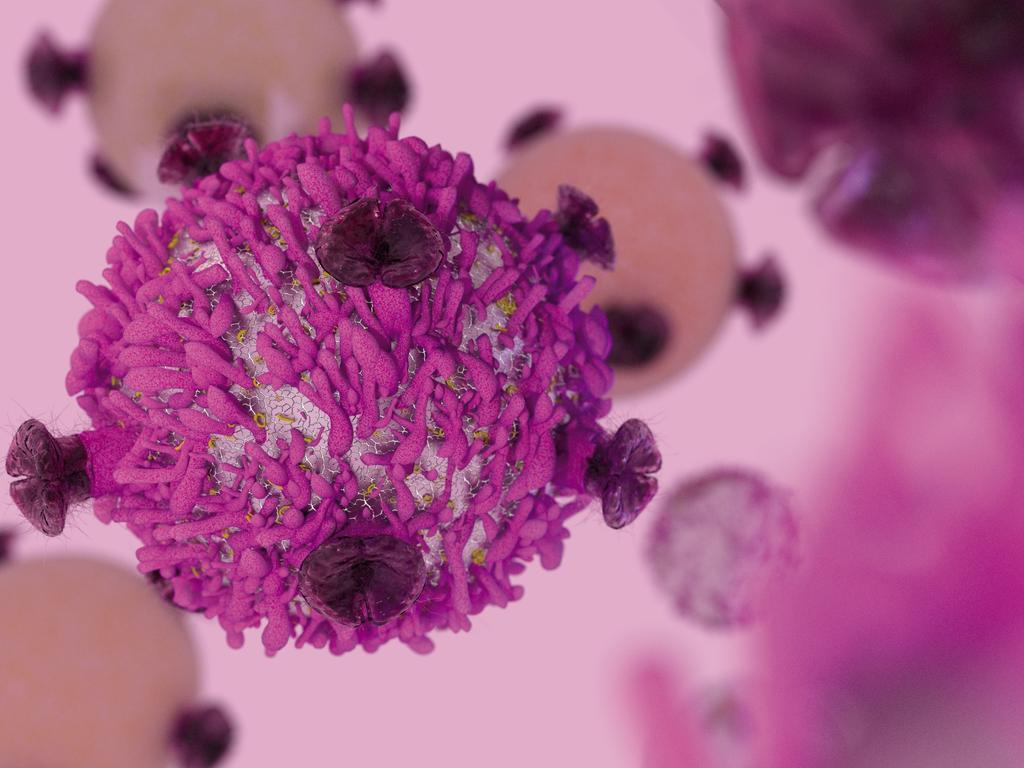
CAR-T cell therapy has been dubbed “the future of cancer therapy” for those who fail conventional treatments. It works by infusing the patient’s own blood, which has been sent to a special laboratory where T-cells are “re-engineered” to carry special structures called chimeric antigen receptors (CARs) on their surface.
When these CAR T-cells are reinjected into the patient, they multiply rapidly and help the patient’s own T-cells identify and attack cancer cells throughout the body.
Multiple myeloma patient Stephen Bambery – whose blood cancer had spread extensively to his bones by the time it was detected last year – received cilta-cel as part of a clinical trial. He is fervently hoping that lives will be saved if the MSAC gives a positive review, as his was.
A year after confronting thoughts of not surviving until his next birthday and not seeing his grand daughters grow up, he is back on the tennis court and golf course. In July 2022, Dr Bambery was selected for a clinical trial of the breakthrough CAR T-cell therapy, which began in October of that year.
“Compared to chemotherapy it was less challenging,” he said.
Dr Bambery said that, during the almost two weeks in hospital for the treatment he felt “reasonable”.
“The biggest benefit is that you don’t have continuing maintenance therapy,” he said.
Now 69, Dr Bambery is in remission and is continuing with bone-strengthening infusions in order to recalcify his bones, which suffered erosion from the condition.
“I’m certainly feeling a level of gratitude. I feel very fortunate that I happened to respond well to the treatments and to the CAR T-cell therapy,” Dr Bambery said.
“It’s just lovely getting back to normal, feeling normal and having time with my family.
“You always think these things happen to other people. Sometimes you are the other person.”
CAR T-cell therapy is considered a medical service rather than a pharmaceutical and requires the commonwealth and respective state and territory governments to fund the therapy on a 50:50 basis.
This can only happen after a positive recommendation from MSAC – a committee and process that the federal Health Minister Mark Butler described as “clunky”. A one-off treatment of cilta-cel costs around $600,000.
The Australian can reveal that globally, 2100 myeloma patients have had their T-cells removed and re-engineered to fight their cancer in the United States and Germany.
The former Liberal government approved and funded a number of CAR-T cell therapies including for several types of lymphoma.
The Albanese government this week provided a $5m grant to a team from Monash University to develop a CAR-T cell therapy for prostate cancer.




To join the conversation, please log in. Don't have an account? Register
Join the conversation, you are commenting as Logout