Budget 2022: PBS-listed gene therapy gives a chance at life
At $3 million per patient it’s the most expensive drug ever listed on the PBS, but for one young family it’s already been priceless.
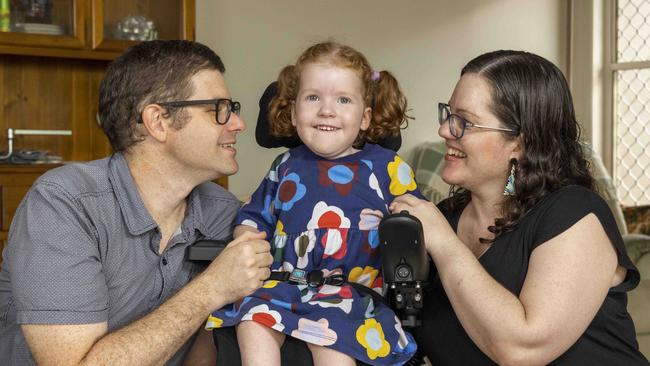
Reena McIntosh was five months old when she was diagnosed with spinal muscular atrophy (SMA), a deadly genetic disease that left her unable to lift her head from her crib and her parents unsure whether she would live to see her first birthday.
“It was really scary, as the parent of a child with SMA. There’s a lot of uncertainty,” said her mother, Katie McIntosh.
Yet Reena not only made it to her first birthday, she has seen her first three thanks to a groundbreaking gene therapy that lets her hold her head up high and sit independently.
On Tuesday, Josh Frydenberg announced a $2.4bn commitment to new and amended Pharmaceutical Benefits Scheme listings that included Zolgensma, the single-dose drug that changed the McIntosh and many other SMA families’ lives.
It is the first gene therapy to be listed on the scheme in our healthcare history, and the most expensive drug to join the list – costing upwards of $3m a patient.
SMA Australia chief executive Julia Cini said the “phenomenal announcement” would give much hope to affected children and their families.
“There are 20 kids each year diagnosed here in Australia with SMA – that’s a whole classroom of children,” Ms Cini said.
Before treatment, one baby would die each month from SMA.
“Having access to treatments like this is game-changing,” Ms Cini said. “It’s completely transformational not only for the immediate family but it changes everyone’s life around them.”
Zolgensma works by targeting the missing or damaged gene and “putting a bandaid over it to make it work like normal”.
Ms Cini said she had seen children go from bedridden to walking and jumping.
It works so well “you could look out into a classroom and you wouldn’t be able to tell which child has SMA”.
However, accessing treatment is only half the battle, she said, and it was equally if not more important for children to be tested for SMA as early as possible.
“The impact of a delayed diagnosis for SMA is pretty devastating,” Ms Cini said.
“A family has enormous guilt when they figure out that they could have had an earlier diagnosis through newborn screening or genetic testing.”
The federal government also set aside an $81.2m package to provide carrier screening for genetic conditions including spinal muscular atrophy.
Ms McIntosh, who is pregnant with the family’s second daughter, said she was relieved to know she and husband Clayton – and other families – didn’t have to punish themselves for delayed diagnosis or to fight for treatment as they did with Reena.
The coming baby has a 25 per cent chance of being diagnosed with SMA.
“Just screening for SMA is so important because every day means loss of function; even if you can’t see it, motor neurons are dying and muscles are dying and we can’t bring those back,” Ms McIntosh said.
“We want families to have access to newborn screenings so they don’t have to watch their child suffer disability that could have been prevented with early diagnosis and treatment.”



To join the conversation, please log in. Don't have an account? Register
Join the conversation, you are commenting as Logout