Global recall of cancer-linked breast implants
The maker of breast implants linked to a rare cancer has been forced to issue a global recall following a significant rise in cases.
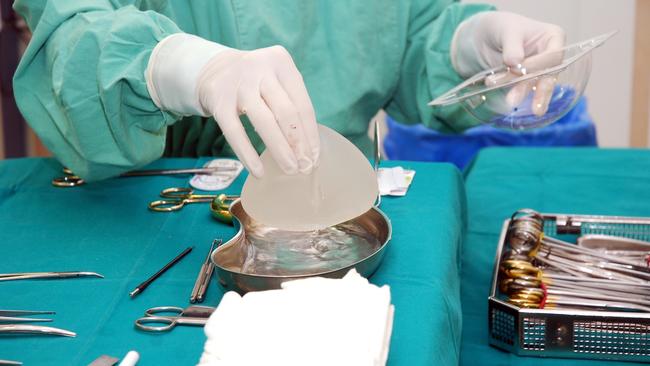
The manufacturer of textured breast implants linked to a rare cancer was yesterday forced to issue a global recall — two weeks after Australia threatened tougher regulatory action — following a significant increase in cases.
Allergan announced the voluntary recall of its Biocell textured breast implants and tissue expanders “as a precaution” following an update on the cancer risk from the US Food and Drug Administration.
Australia’s Therapeutic Goods Administration was already aware of the links to a cancer known as breast implant-associated anaplastic large cell lymphoma (BIA-ALCL) and consulted industry officials and health experts on a proposed crackdown.
The FDA has now tallied 573 unique cases globally of BIA-ALCL and 33 patient deaths, including some Australians, which it acknowledged was significantly higher than its previous update. With 481 of the cases linked to Allergan implants — including 12 of 13 deaths where the manufacturer was known — the FDA asked the manufacturer to issue a recall.
“Although the overall incidence of BIA-ALCL appears to be relatively low, once the evidence indicated that a specific manufacturer’s product appeared to be directly linked to significant patient harm, including death, the FDA took action to alert the firm to new evidence indicating a recall is warranted to protect women’s health,” said FDA principal deputy commissioner Amy Abernethy.
Allergan had previously stood by its products, even as French regulators banned the implants and other jurisdictions looked to follow suit.
The TGA had closed its final round of consultation when the FDA gave its update, and Allergan issued the recall; however, the proposed Australian response would cover a broader range of products and manufacturers.
“BIA-ALCL is more likely to occur in rougher-surfaced implants, and the TGA is proposing to either cancel or suspend particular products,” the regulator said recently.
“There are alternative smooth implants available and these have not been associated with known cases of ALCL in Australia.
“However, because the risk to women who already have these implants is very low, experts do not recommend that women who already have them need to have them removed unless there is a confirmed diagnosis of ALCL.” Plastic surgeons have warned that the debate over safety risks and threat of regulatory action are causing anxiety among women who have the implants and might be concerned for their safety. It is not known how many women are affected.
BIA-ALCL is not a breast cancer as such, but a cancer that grows in the fluid and scar tissue that forms around a breast implant or, less commonly, in the breast or armpit. The risk is rare — experts suggest between one in 1000 and one in 10,000 — and it has been suggested women who are diagnosed can have the implant removed and be cured.
The director of the FDA’s Centre for Devices and Radiological Health, Jeff Shuren, reiterated the FDA did “not recommend removal for patients without symptoms due to potential risks”.
In a statement, Allergan, which is also a pharmaceutical company with its headquarters in Ireland, said “patient safety is a priority”. It emphasised that no regulators had recommended the removal or replacement of textured breast implants.
“Patients are advised to speak with their plastic surgeon about the risks and benefits of their implant type should they have any concerns,” the statement said.



To join the conversation, please log in. Don't have an account? Register
Join the conversation, you are commenting as Logout