‘Disappointment’ at rejection of new dementia drug
Australia’s drug regulator has decided not to allow a drug recently approved in the UK and marketed in the US as slowing cognitive decline.
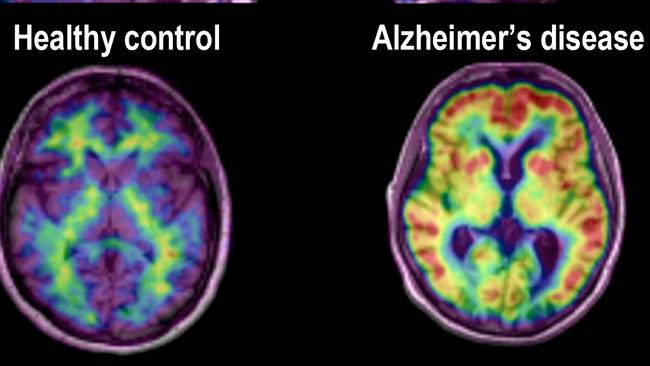
Dementia Australia has expressed disappointment at the decision to reject for use in Australia a new drug claimed to slow the progress of Alzheimer’s disease.
The Therapeutic Goods Administration on Wednesday announced its decision not to register lecanemab for the treatment of patients with mild cognitive impairment because of Alzheimer’s disease and mild Alzheimer’s dementia.
The drug was given the green light for use in Britain in August, for private prescriptions only, and has also been approved for use in the US but not in Europe.
Eisai, the pharmaceutical company that developed the drug, has said it would ask the TGA to reconsider the decision.
Lecanemab worked by removing amyloid plaques from the brain and in doing so slowed cognitive decline associated with the disease, said Dementia Australia, the peak body for those living with dementia.
The TGA said the efficacy of the drug did not outweigh safety risks associated with its use.
“In particular, the TGA delegate considered the frequent occurrence of amyloid-related imaging abnormalities (ARIA)” in patients treated with the drug, it said.
In Britain, the medicine has been hailed as a “potential wonder drug”, with trials showing it could slow progression of the disease, although caution has been expressed about whether it is useful for only some patients.
The European Medicines Agency rejected a licence for the drug in July, citing a risk of bleeding and swelling in the brain.
That decision was criticised by Alzheimer Europe.
Eisai said lecanemab was approved in Japan, China, South Korea, Hong Kong, Israel and the UAE, and was being marketed in the US, Japan and China.
Dementia Australia chief executive Tanya Buchanan said the decision would deprive Australians of the choice to access the potential benefits of the new treatment. “While we respect the TGA as Australia’s medicines regulator, should this decision be upheld it will be a blow to Australians who may be able to benefit from lecanemab. Dementia Australia is disappointed that Australians living with Alzheimer’s disease in its early stages may be unable to access the same choice of treatments as people living in other countries,” Professor Buchanan said.
“Alzheimer’s disease is a progressive and ultimately fatal neurological condition so slowing decline when people are experiencing mild symptoms is incredibly important in supporting people to maintain quality of life for longer.
“Lecanemab is not a cure and is not for all people with a diagnosis of Alzheimer’s disease. Like many medicines, it comes with some significant risks. It is, however, widely seen as a historic first step towards reducing the huge impact of Alzheimer’s disease and for people living with the condition it signified hope.”




To join the conversation, please log in. Don't have an account? Register
Join the conversation, you are commenting as Logout