Coronavirus: No reason to pause AstraZeneca vaccine administration in Australia: TGA
Australia to push ahead with COVID-19 vaccination program using AstraZeneca jab as health officials dismiss blood clot fears.
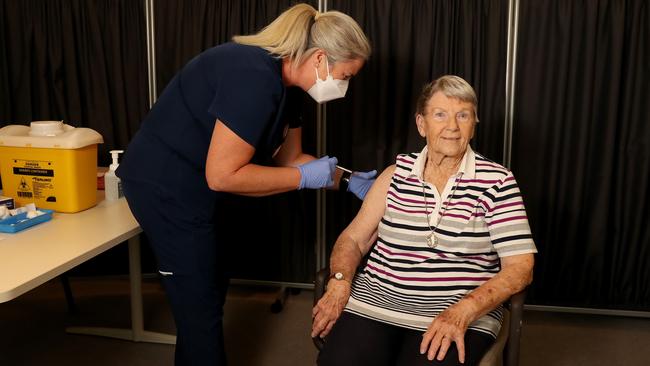
Australia will push ahead with its COVID-19 vaccination program using the AstraZeneca jab, as senior health officials dismiss concerns over 37 bloodclotting incidents out of 17 million inoculations in Europe that have sparked temporary suspensions of the program.
France, Germany, Spain, Italy and Indonesia were the latest countries to suspend their AstraZeneca programs on Tuesday pending a review by European medical regulators.
Australian officials are looking to the United Kingdom which has delivered the most jabs of the vaccine. The UK is continuing its program, insisting there has been no link to bloodclotting in the 11 million jabs administered so far.
AstraZeneca conducted a review of its data following the suspensions in Europe and said it had found fewer cases of blood clot disorders in people who had received a jab than in the general population.
Australia’s Therapeutic Goods Administration said it was watching the results of the review by the European Medicines Agency , but dismissed any link between the vaccine and blood clotting as biologically implausible based on the available evidence.
AstraZeneca vaccinations — which make up the bulk of Australia’s program with 54 million doses being manufactured locally by CSL Ltd — are being given to quarantine and border workers, frontline healthcare staff and the elderly in nursing homes.
Australian health chiefs said no cases of blood clots had been observed in Australia.
University of Queensland associate professor Paul Griffin, who runs clinical trials of COVID-19 vaccines, labelled the suspension of AstraZeneca by European countries an over-reaction. “If there was a safety concern I’m sure the TGA would have no hesitation in slowing down the rollout,” Professor Griffin said. “I think this is a situation that has been incited by a country that was overreacting a little bit, and then the others, in wanting to be perceived to be demonstrating an abundance of caution, followed suit.”
Professor Griffin said he was concerned that the European suspensions may damage confidence in the vaccine. “I think it’s especially important to highlight how rigorously our regulator is reviewing the data,” he said. “They are certainly reviewing lots of data in real time.”
Scott Morrison said on Tuesday Australians should listen to the official advice from the country’s medical experts as he called on his colleagues to support the rollout and not “seek to undermine it”.
“I think it is incumbent on all of us to be supporting that vaccination program,” the Prime Minister said. “All of us should be doing that, Mr Speaker, not trying to seek to undermine it by making false claims about rollouts.”
The Prime Minister’s comments came after Nationals senator Matt Canavan, crossbencher Craig Kelly and One Nation’s Pauline Hanson called for the vaccine’s use to be suspended while testing was conducted.
Chief Medical Officer Paul Kelly said more Australians had “drifted towards” receiving the jab since the rollout began, but warned he “wouldn’t be surprised” if some Australians were more hesitant to take AstraZeneca following the European suspensions. Health Minister Greg Hunt said the Morrison government “unequivocally” supported the AstraZeneca jab.
The TGA has analysed data on all adverse events until March 8, covering every country administering AstraZeneca. It said it was satisfied there had not been an increase in cases of thrombosis or pulmonary embolism beyond what would normally be expected to occur in any given population.
“The TGA does not have any evidence of a biologically ¬¬ plausible relationship that could suggest a cause-and-effect relationship between vaccination and blood clots,” the regulator said.
“The European Medicines Agency continues to investigate the issue and the EMA remains of the view that the benefits of the AstraZeneca vaccine in preventing COVID-19, with its associated risk of hospitalisation and death, outweigh the risks of side effects.”
There was debate in the Coalition party room on Tuesday as to why Australia was not pausing the AstraZeneca rollout for a few days until the EMA made its determination on Thursday.
Labor leader Anthony Albanese accused Senator Canavan of undermining public confidence in vaccines.
The UK has opted not to halt its rollout, with the Medicines and Healthcare Products Regulatory Agency urging people to continue to receive the jab.
“We are closely reviewing reports but given the large number of doses administered, and the frequency at which blood clots can occur naturally, the evidence available does not suggest the vaccine is the cause,” said Phil Bryan, the vaccines safety lead at the MHRA.
The AstraZeneca vaccine was temporarily suspended in Denmark last week after the death of a 60-year-old woman who formed a blood clot shortly after she had been vaccinated and died. Austria had earlier stopped using a batch of AstraZeneca shots while investigating a death from a coagulation disorder and an illness from a pulmonary embolism.




To join the conversation, please log in. Don't have an account? Register
Join the conversation, you are commenting as Logout