How Donald Trump beat Covid-19 with an experimental cocktail of drugs
Doctors ‘threw the kitchen sink’ at Donald Trump when treating him for the virus. Researchers are trying to work out how to treat the rest of us.

When US President Donald Trump contracted coronavirus, many were pessimistic about his chances of making a quick recovery. At 74, Trump’s risk of death was elevated. He is 190cm tall and weighs 111kg, putting him in the obese range, another risk factor for severe disease.
When Trump appeared to make a speedy recovery from COVID-19 after spending only three days in hospital, he credited an experimental antibody cocktail with his return to health and proclaimed that he wanted all Americans who fell ill with coronavirus to be given the same treatment.
It’s impossible to know whether the investigational antibody cocktail that Trump was given — developed by US biotech Regeneron Pharmaceuticals — reduced the severity of the President’s illness. Trump also took the corticosteroid dexamethasone, and the antiviral drug remdesivir, as well as zinc, vitamin D, the heartburn medication famotidine, melatonin and aspirin.
“They threw the kitchen sink at the guy,” says Australian immunologist John Dwyer.
There’s good evidence that dexamethasone reduces mortality rates, and some evidence that remdesivir reduces illness duration by a modest amount. Antibody treatments have shown promising results in test tube studies, but clinical trials are yet to prove the efficacy of the cutting-edge therapy.
And despite Trump’s pronouncement that he wanted all Americans with coronavirus to have access to antibody treatments, even if clinical trials were to prove successful, it would be prohibitively expensive, and practically impossible, to roll out such treatment on a mass scale.

“On a commercial basis it would be thousands of dollars for a course for this stuff,” says Dwyer, an emeritus professor of medicine at the University of NSW.
“It is not possible to mass-produce these antibodies to give to millions and millions of people. To grow the little tiny cells to produce a whole host of proteins is a laborious process, and you only get a small amount of production from any individual cell. You couldn’t possibly produce enough of it to give like you would a vaccine, to everybody.
“Plus the half life of these antibody cocktails is only 21 days anyway, so it certainly doesn’t produce long-lasting immunity.”
Dwyer says if antibody treatments were to be provided to ordinary people who fell ill with COVID-19, they would have to be reserved for the sickest and most vulnerable. So how exactly do they work?
“On the COVID virus, there is a particular area called the spike protein, which enables the virus to bind to receptors in the lining of the mouth and the nose and the eyes and that’s how it locks on to a human cell and gets inside,” Dwyer says.
“So if you’re infected with the virus you’ll make antibodies to maybe 50 different parts of the virus. None of them are important except the antibodies you make to the spike protein.
“So we call that a monoclonal antibody, mono meaning it only recognises one tiny part of the virus. If you take a type of cell called a bone marrow-derived B cell, and you grow these cells up in culture, the only product of these particular B cells is an antibody that binds to that spike protein.
“So what people have been doing with the Regeneron therapy is to produce monoclonal antibodies just to two parts of the spike protein. And if you can give antibodies to someone, those will bind onto the virus in the bloodstream and stop them infecting cells. That’s the theory.”
As studies continue into monoclonal antibody therapies, clinical trials have begun to deliver results on a host of other potential treatments.
When the first cases of COVID-19 began to appear in hospitals around the world, the only treatment the doctors were reliably able to provide was supportive care — helping patients to breathe by giving oxygen, and administering paracetamol to reduce fever.
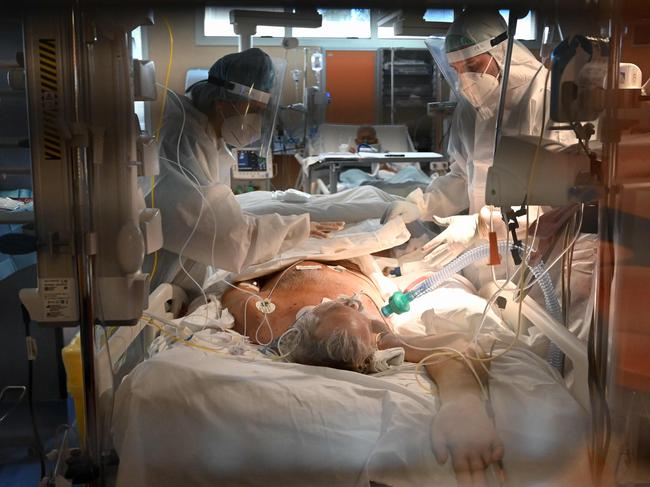
Many hospitals also administered antiviral drugs experimentally with no idea whether they would have any effect.
Ten months on from when the first cases of the novel coronavirus began to be seen in Wuhan, scientific evidence of effective treatments still remains scant. However, hundreds of clinical trials are under way throughout the world, testing whether existing drugs can be repurposed to treat the coronavirus, and new drugs are being developed that are specifically tailored to fight COVID-19.
Despite the scientific effort, only one major gold-standard randomised clinical trial has so far delivered firm evidence of the efficacy of a drug treatment. In June, the RECOVERY trial involving every hospital in the UK reported that dexamethasone markedly reduced mortality rates in patients suffering severe COVID-19.
In those who were receiving supplemental oxygen, the study found death rates were reduced by 20 per cent in those who took the drug. And in those requiring invasive ventilation, mortality rates were reduced by an impressive 35 per cent when dexamethasone was administered.
Australia’s National COVID-19 Clinical Evidence Taskforce, which develops evidence-based clinical guidelines for clinicians on treating COVID-19, has now recommended that dexamethasone be administered intravenously or orally for up to 10 days in all adults with COVID-19 who are receiving oxygen, and the drug is now standard treatment in Australian hospitals for all oxygen-dependent patients.
The other treatment that has been used in hospitals in Australia is another one of the drugs that Trump took, the antiviral remdesivir. This was the first drug given provisional approval by the Therapeutic Goods Administration to treat COVID-19.
Remdesivir was developed as an antiviral to fight Ebola, and results from clinical trials have found that it reduces a patient’s average recovery time from 15 to 11 days.

However, a major clinical trial conducted by the World Health Organisation recently found that remdesivir had no effect on mortality rates.
The Solidarity Trial reported a similar lack of efficacy for three other repurposed drugs — the antimalarial hydroxychloroquine, the HIV drug lopinavir and the auto-immune drug interferon.
The director of Melbourne’s Peter Doherty Institute for Infection and Immunity, Sharon Lewin, likens remdesivir to the early HIV drugs that showed a modest effect but were eventually replaced by much more effective antiviral therapies.
“I think remdesivir looks like a pretty crappy drug, really,” Lewin said.
“It shows modest efficacy as an antiviral, and it might just be that we’re not using it at the right time, we’re using it when people are too sick. But it’s intravenous and costly.”
Despite hydroxychloroquine’s lack of success as a treatment, trials are continuing into whether the drug could be effective in preventing COVID-19 in those who are exposed to the virus.
Doctors are eagerly awaiting the results of ongoing clinical trials into a wide range of other drugs that have been repurposed to combat COVID-19, as well as those specifically designed to fight the virus.
The therapies fall into three main categories:
• Drugs being trialled that target the virus to reduce its ability to replicate in host cells.
• Immunomodulatory and anti-inflammatory therapies that suppress the problematic hyper-immune response associated with coronavirus.
• Medications that address the life-threatening complications of COVID-19.
“What’s remarkable about COVID-19 is just how fast the research field has moved since the start of the year,” said Joseph Doyle, a clinician at Melbourne’s Alfred Hospital and a researcher at the Burnet Institute.
“What would normally take years to go from a new drug product through to something in human trials is now progressing not in years but in months.”
Lewin describes research into the use of designer antibody drugs and other small molecule inhibitors as one of the most exciting areas of research.
Hyperimmune globulin and convalescent plasma therapies are other antibody therapies that are being trialled.
Dwyer also nominates stem cell research as a promising field.
“We’re pretty certain that most people who die from acute COVID-19 infections do so because the virus produces such a vigorous immune response that we sort of self-destruct,” Dwyer says.
“We talk about an immunological storm descending on people.”

Researchers are working on growing cells from stem cells that would have the capacity to regulate the immune system, Dwyer says.
“If you like, these are the immunological policemen that patrol around our body and turn off the immune response. This stem cell research could have the capacity to suppress an inappropriately enthusiastic immune response.”
While much of the global attention focuses on the race to find an effective vaccine against COVID-19, Lewin believes the chances of having a suite of therapies available in coming months and years that can prevent death and severe illness is high.
“I’m quite confident about that,” she says.
“I’m most inspired by HIV. The whole HIV epidemic was turned around just with treatment, without a vaccine. As we learn more about how the virus causes disease, we’ll get better at tailoring treatments. I’m sure about that.”




To join the conversation, please log in. Don't have an account? Register
Join the conversation, you are commenting as Logout