Poorest die as diabetes drugs hoarded in cities
Australia is failing to ensure that new-generation drugs that could help prevent a devastating tide of diabetes complications are made available to the sickest in the country.
Australia is failing to ensure that new generation drugs that could help prevent a devastating tide of diabetes complications, including renal failure, amputations and blindness, are made available to the sickest people in the country.
Top doctors have joined with one of the world’s biggest pharmaceutical companies in calling for new diabetes drugs, including Mounjaro and Ozempic, to be “ringfenced” for priority populations such as Aboriginal diabetics in remote Australia.
Currently, drug shortages and exploding demand in the cities, combined with the fact that the drugs are not licensed or funded to treat obesity, means that disadvantaged populations in the Northern Territory and Western Australia are unable to access the new drugs at all, despite being in desperate need.
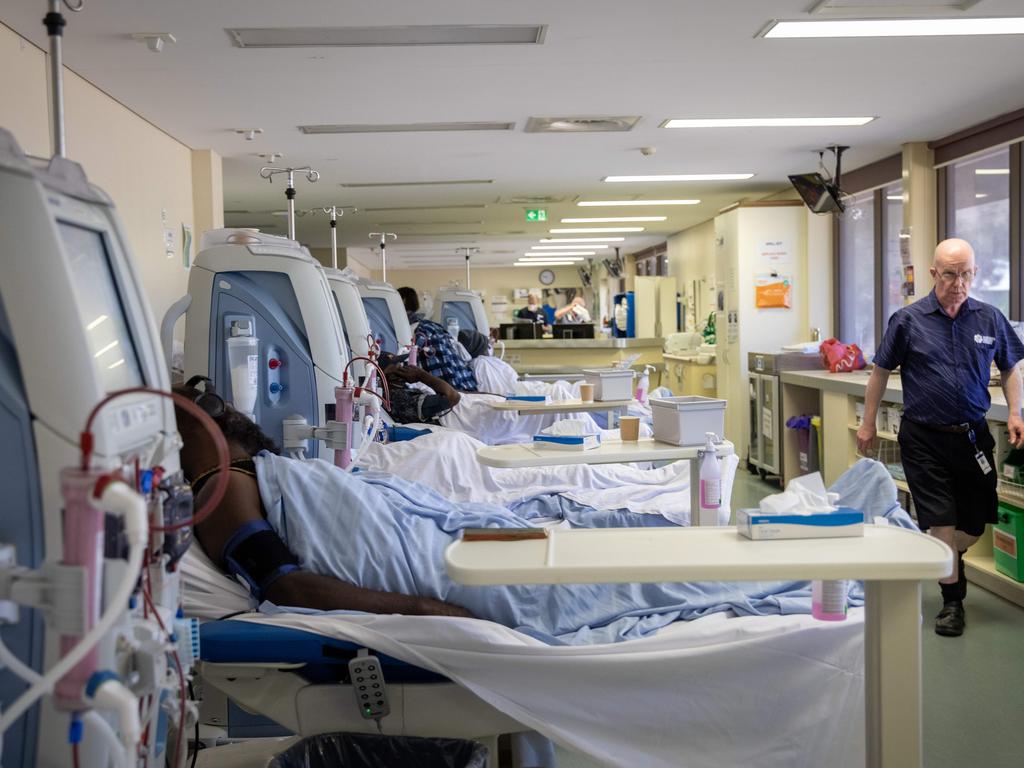
The Central Australian Aboriginal Congress is begging for priority access to the new drugs, known as GLP-1 agonists, as renal clinics reach capacity in Alice Springs and a burgeoning epidemic of type 2 diabetes hits Aboriginal children as young as 4.
“The Australian government should ensure that all Aboriginal people have affordable access to GLP-1 receptor agonist medication, including by listing these medicines on the Pharmaceutical Benefits Scheme and in the highly specialised drugs program, for both diabetes and obesity formulations,” Congress chief executive Donna Ah Chee said.
“Additionally, in times of shortage, access to GLP-1 receptor agonist medications should be prioritised for Aboriginal people especially in remote and very remote areas.”
Top doctors and policy heads from the global pharma giant Eli Lilly will give evidence on Friday at a federal parliamentary inquiry into diabetes. Its senior vice-president of international medical affairs for obesity and diabetes Rachel Batterham, who has flown to Canberra from London to give evidence, has spoken openly of her shock at the extent of the type 2 diabetes epidemic in remote Australia.
The chronic disease is pushing hospitals to the brink of collapse, with up to a third of patients in urban centres affected by the condition. Four in 10 Aboriginal people in Central Australia have diabetes and the region has the highest rate of renal dialysis per head of population in the world.
“I have to say, I’ve (been) shocked to be honest, in a first-world country, how poor the level of glycemic control is in Australia,” said Dr Batterham, a global clinical leader in obesity and diabetes who established the Bariatric Centre for Weight Management and Metabolic Surgery at University College London Hospital’s NHS foundation trust.
“In the UK, these drugs are available so that we can get people’s glucose under control, and then the complications come down. It’s a cost now to save later.
“We need to ensure that these medications get to the people who really need them. There are mechanisms where medications can be ring-fenced for the people with the greatest need. And I would have thought that that would be possible.”
Eli Lilly manufactures Mounjaro, a new-generation diabetes and obesity drug that combines glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonists in a single molecule.
Chairman of the diabetes inquiry, Labor MP Mike Freelander, backed the call for priority access for Aboriginal people in remote Australia.
“There is no doubt that type 2 diabetes, whilst it’s a problem across the whole country, there’s no question that Indigenous communities are really suffering the most, and access to care and medications is very difficult,” he said.
“The new GLP-1 agonist medications and some of the other newer medications are gamechangers, but only if regular supply is available.
“When we have supply shortages it is a major concern that the wealthier people can access it and the poorer people can’t and the poor need it the most.”
Eli Lilly has hit out at Australia’s sclerotic drug approvals process that has meant drugs such as Mounjaro and Ozempic have been knocked back for obesity despite extraordinarily high rates in Australia. “Access to new innovative medications seems to be a particular barrier here, compared to say the UK … where the National Institute for Health Care and Excellence looks at the cost effectiveness of medications, and if it’s cost effective, then they approve it.”
Barriers to access and funding are being examined in a federal inquiry into medical technology.




To join the conversation, please log in. Don't have an account? Register
Join the conversation, you are commenting as Logout