It’s revolutionised blood cancer treatment. Could CAR T-cell therapy be used for tumours?
For years, scientists have been trying to figure out how to apply a last-line blood cancer treatment to solid tumours. New findings have them moving closer.

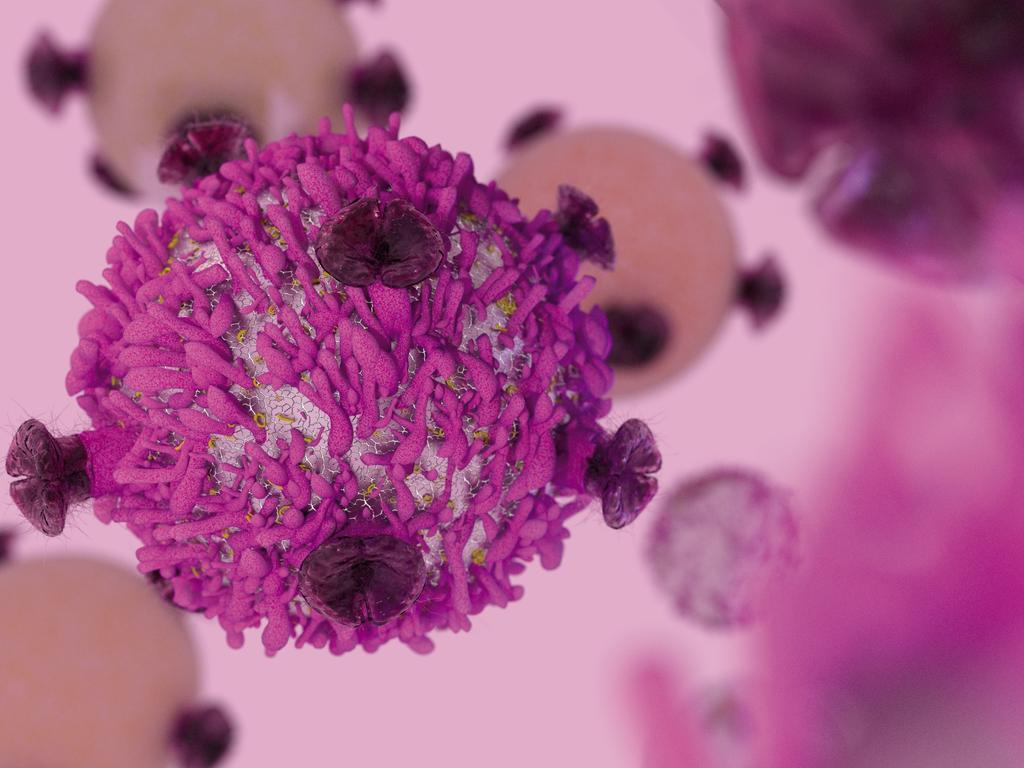
A form of personalised immunotherapy has proven highly effective at curing certain blood cancers when other treatments have failed, but scientists have struggled to get CAR T-cell therapy, which re-engineers a patient’s own T-cells to kill cancer cells, to work on solid tumours without triggering a toxic response.
Now, Australian scientists from the Peter MacCallum Cancer Centre have made an early, but significant discovery they hope could change that.
Associate professor Paul Beavis, who co-led the study, said his team had used genetic engineering to activate cancer-fighting immune proteins but only at the site of the tumour. It has allowed them to effectively treat solid cancers using CAR Ts-cell therapy without triggering the usual toxicity that’s proved problematic in the past and resulted in the cancer-fighting cells being rejected. They did so with the help of the gene-editing tool CRISPR.

The theory has only been tested in pre-clinical animal trials, but the results have been so effective scientists hope they could move to human trials in the next five years. The findings have been published in the latest edition of the prestigious journal Nature.
“These engineered cells can now deliver immune-activating molecules directly to the tumour, sparing healthy tissue and avoiding the toxic side-effects often seen with CAR T-cell therapy,” Professor Beavis said.
“We identified two key promoters, which are active in CAR T-cells primarily in tumours. By using CRISPR we can insert genes for powerful cytokines such as IL-12 and IL-2 under the control of these promoters, which resulted in a localised and potent immune response against cancer.
“We tested this new technique in multiple cancer models such as breast, colon and ovarian cancer and the results were incredibly promising with cure rates close to 100 per cent.”
While there is still a lot more research, funding, and years of work needed before the therapy can move to human trials, the results have filled scientists with optimism. One of those is professor Phil Darcy, another of the study’s co-leads.
“As the system we have developed allows for the immune response to be localised to the tumour microenvironment we see less toxicity, which should result in a safer treatment,” he said.
“In animal models, this targeted strategy led to improved tumour control and long-term survival, even in models where human cancers were introduced.”
Mice are often used in science to test early stage theories before they then advance to human trials for both safety reasons and because mice and humans share some similar physiological and genetic characteristics, which can act as early guideposts.


If three phases of human trials prove successful, the therapy would then need to be approved for use before coming to market.
“If it’s approved, it costs around about half a million dollars, which is comparable to other forms of immunotherapy,” Professor Darcy said. “But we’re also looking to develop other … off-the-shelf therapies as well that we can apply this type of technology to, and that would considerably reduce the cost further. It’s only been globally available since 2017 but I think The technology is advancing rapidly and the goal is to make this therapy available to everybody that needs it.”
The study was undertaken with financial support from the Cancer Research Institute, the National Health and Medical Research Council and the National Breast Cancer Foundation.




To join the conversation, please log in. Don't have an account? Register
Join the conversation, you are commenting as Logout