Doctors call for national shake-up of cosmetic injectables industry
‘Something has to change’: Doctors ask what will it take before regulators act on our cosmetic injectables industry? | WATCH

As the elevator doors open to the second level of an office building in Sydney’s bustling city centre, you are greeted by the unmistakable but somewhat pungent scent of herbal medicine.
The doors open to an ageing and almost ghostly quiet floor that’s lined with small, office-like rooms. A young woman scrolls through her phone as she sits patiently on a chair in a communal waiting room. Each office contains a small business, many have signs indicating they are medicine-related, including a dentist.
Walk down a small corridor and you find an unremarkable single door leading to Suite 204. There is no sign out the front or any other indication of the horrendous treatment that allegedly happened in the room. This is the location of the latest raid on an unauthorised cosmetic injectables clinic.
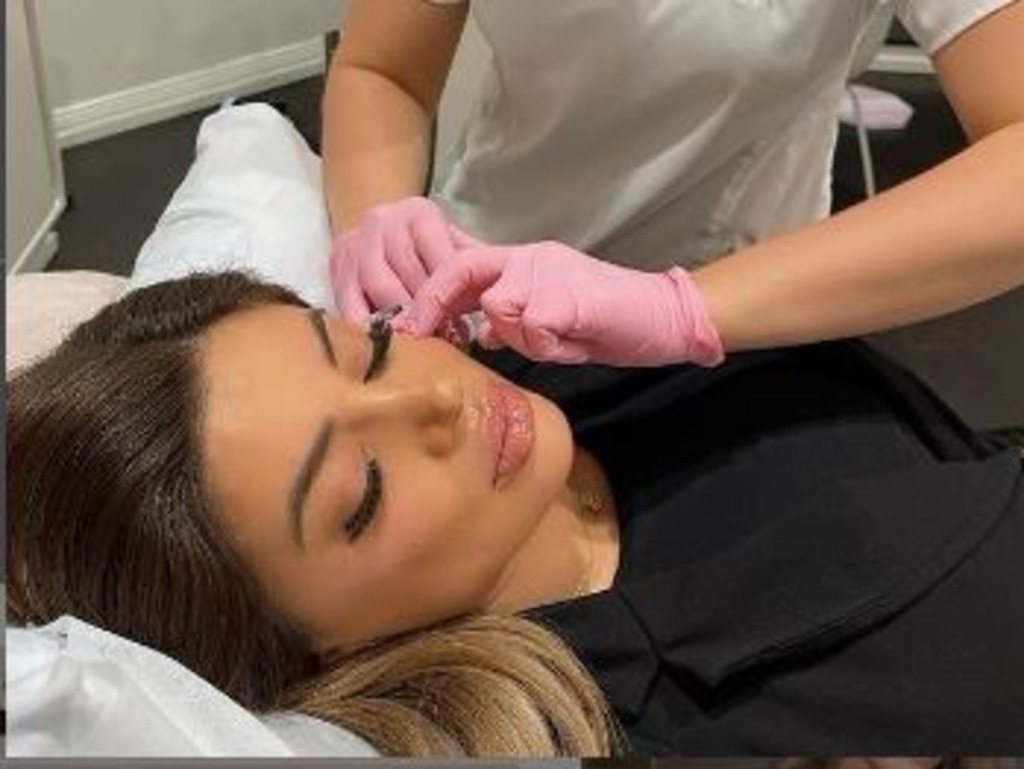
It’s a far cry from the tranquil images of beautiful women and words of assurance shown on the clinic’s now-shuttered website.
“Here at Ketthip Beauty Clinic you will receive personal treatment plan that combines leading-edge procedures with sound medical practices,” an old snapshot of the website states.
“We provide a range of treatments that are backed by research and proven results. Our treatments use high-quality medical products, cutting-edge equipment and technology that are FDA and HAS approved.”


This week the Health Care Complaints Commission issued a public warning for patients of Ketthip Beauty Clinic, who may have been exposed to bloodborne viruses such as hepatitis C or HIV.
The NSW agency alleged staff were pretending to be trained and accredited above their skill level; that invasive procedures – such as breast augmentations – were being completed by non-registered practitioners; surgical tools only intended to be used by veterinarians were seized; and a large quantity of cosmetic injectables were also found – some of which were imported and past their expiry.
The HCCC issued an interim prohibition order for I Pei Pan which prohibits her from performing any cosmetic treatments under any circumstances.
The Ketthip Beauty Clinic case is alarming, and the risk to patient safety is significant and cannot be ignored. The HCCC believes at least one patient may have experienced an adverse reaction to procedures performed at the clinic.
What happened in Suite 204, if proven true, is shocking. But it raises the question: what else is happening in Australia’s cosmetic injectables industry that regulators are not aware of?
The incident has renewed calls for sweeping changes to Australia’s cosmetic injectables industry and for the rollout of new guidelines to be sped up.
“The regulator absolutely needs to act,” says specialist plastic surgeon Dr David Morgan, who is also the president of the Australian Society of Plastic Surgeons.
Medical professionals, including those who work in the cosmetics industry, say federal regulations and state enforcement of the rules are too lax and it is creating an environment where poor practices are happening.
In late January, three people in NSW were hospitalised with the rare but potentially fatal condition, botulism. It is suspected they were given an unregulated and high-dose version of an anti-wrinkle injectable in a backyard operation in Sydney. One of the patients was so sick they were treated in intensive care. The woman accused of administering the dodgy injections is also believed to have been working in Victoria and was advertising her services via WhatsApp.
In October 2024,anothera public health warning was issued for a cosmetic clinic in Sydney, where it was feared patients may have been exposed to bloodborne viruses such as hepatitis B, hepatitis C or HIV due to infection control breaches.
As previously reported, counterfeit and illegally imported versions of cosmetic injectables are also circulating in Australia. The Therapeutic Goods Administration says it has received more than “99 referrals from the Australian Border Force in relation to intercepting therapeutic goods categorised as cosmetic injectables at the border”.
The HCCC has confirmed it continues to see alarming cases where unqualified individuals operate outside the law, using unsafe practices and unapproved medications.
“We have serious concerns about infection risks, the use of expired and imported medications, and unregistered individuals impersonating medical professionals,” a spokesman for the HCCC says.
“Our immediate priority is public safety and addressing the dangers posed by some non-registered practitioners.”
In September 2023, the Medical Board of Australia and the The Australian Health Practitioner Regulation Agency announced they were turning their attention to cosmetic injectables.
Dr Morgan says he and many of those working in the medical industry have been raising the alarm about the sector for years and he cannot understand why reform has been so slow.
“The recent high-profile incidents are just further examples of what we know is happening out there already. I don’t think we need any more examples to make everyone realise that something has to change,” he says.

Dr Ritu Gupta is an accredited specialist cosmetic dermatologist who runs a clinic in Sydney.
“Where my practice is in Sydney, in Ultimo, I am literally down the road from where a woman died a couple of years ago because a Chinese doctor came on a tourist visa and administered local anaesthetic intravenously for injecting filler into her breast, and (the patient) had a cardiac arrest and died,” she says.
Jie Shao was later found guilty of manslaughter over the incident and sentenced to almost seven years in prison.
“It is not until adverse events like this happen, like where three women with botulism and people being exposed to lethal diseases, that our regulators step in.”
Some doctors want to see changes to federal regulations to make a person’s qualifications more obvious to patients, for there to be new minimum training standards for injectors, and for there to be a safeguarding of certain titles, similar to the restrictions on the term “cosmetic surgeon”.
Other medical professionals are also flagging concerns. In a complaint allegedly sent to federal and state regulators and to The Australian, allegations have been made about multiple breaches of the rules and regulations governing the sector.
The complaint details eight issues and alleges that, in some instances, non-prescribing nurses have created prescriber accounts using the details of a doctor, allowing them to order and purchase S4 medications, such as anti-wrinkle injections and filler, without oversight.
If correct, such a move would be a breach of the Therapeutic Goods Act. It could also compromise the insurance and accreditation covering those involved.
No medical professional quoted in this story is part of that complaint. But those calling for change worry that poor practices could be creeping deeper into the industry and they consider a tightening of the rules the only way to rein things in.
“What we’re increasingly finding in this industry is that more and more people are moving into it with limited knowledge. And if it all goes well, it’s fine, but if suddenly something happens that isn’t in the normal process, then they are well out of their depth, and the patient will suffer,” Dr Morgan says.

Typically, non-surgical cosmetic procedures include things such as chemical peels, laser, microdermabrasion, anti-wrinkle injections and filler. Dr Gupta has provided corrective care to patients who have experienced disturbing effects from such treatments elsewhere.
“I’ve treated a woman who, half her forehead skin has died, what we call necrosis,” she says. “She had fat injections into her forehead in Korea, which had blocked off two arteries which, anyone with any anatomical knowledge would never inject.
“I’ve treated another woman who went to some backyard provider in a home and she had big lumps of infection all over her face at every injection point where she had some sort of filler injected. I don’t know what it was, and they grew E. coli, which is bacteria that is associated with faeces – that’s faecal contamination; the mind just boggles.”
One of the hurdles to changeis the way states and the commonwealth interact; legislation for industries such as cosmetic injectables is made federally. Each state and territory then interprets the legislation independently and is responsible for enforcing it, meaning there is no national consistency.
Regulation of products and workforces is also divided between state and federal agencies as well as professional bodies. All of which makes for a bureaucratic mess.
Nonetheless, AHPRA and the national medical boards are now working to develop new guidelines for the industry. Doctors worry those new guidelines are taking too long to arrive.
“Those guidelines were meant to have been released some time ago for health practitioners, which includes doctors but is really referring to non-doctors – so nursing staff, etc – who practise non-surgical procedures and advertise non-surgical procedures,” Dr Morgan says.
“But those haven’t been brought in and I think part of the problem is that it’s such an enormous industry with so many different practitioners involved in it, that (those setting the rules) are trying to figure out how and where to draw the line and who’s responsible for controlling them, if you like.”

Dr Gupta agrees swifter change is required. “I think that whatever that delay is, they should delay no further,” she says.
A spokesman for AHPRA and the national medical boards says they are close to implementing updated national guidelines around non-surgical cosmetic procedures.
“The proposed guidelines have been developed after the review of extensive feedback received during the consultation phase, which raised a wide range of complex, diverse and, in some cases, polarised views,” the spokesman says.
“As the guidelines will cover a vast range of cosmetic procedures, ranging from those with higher risks through to very low risk, they required an appropriate amount of time and consideration to identify the right approach to cater for the risks identified.”
He also says the groups are concerned about practitioners using new models of care, including telehealth, in ways that may be putting profit ahead of patient welfare and taking advantage of consumer demand for certain treatments. He also pointed to several changes to improve patient safety, including the introduction of the taskforces and hotlines established following an investigation into more invasive cosmetic surgery.
“Since then, we have received over 1500 calls and received over 500 notifications related to cosmetic surgical and non-surgical procedures,” the spokesman says.
“In response to the notifications, AHPRA and national boards have taken regulatory action in relation to 35 practitioners. This includes 20 practitioners where the concerns related to non-surgical procedures such as cosmetic injectables.
“Most notifications have related to doctors, and around 15 per cent related to nurses.”
There has been some movement already. In December, Queensland Health sought to clarify its interpretation of the federal legislation covering non-surgical procedures. In doing so, it flagged stricter rules including around the purchasing and prescribing of medications used in cosmetics. At the time, the department said the rules were not new, it was simply reiterating existing rules. That came as a surprise to many, including Dr Morgan who says he has held discussions with health officials there about the changes.
“There are hundreds if not thousands of clinics that are effectively nurse-led that are run by businesses that don’t follow these guidelines. And it was a great surprise to Queensland Health to find out that that was what was happening,” Dr Morgan says.
“In our view, the regulator’s role is to be aware of what is happening in the industry and that space and make sure that people are compliant with existing regulations.”
Dermatologist Dr Ritu Gupta is a columnist for The Australian.
Know more about this story? Contact the reporter: pennytimms@protonmail.com




To join the conversation, please log in. Don't have an account? Register
Join the conversation, you are commenting as Logout