COVID-19 vaccines: What’s coming and when?
As countries and companies race to produce a safe and effective coronavirus vaccine, here’s a guide to the frontrunners.

Some 170 COVID-19 vaccines are in development around the world, according to the World Health Organisation, each one promising to protect people from the deadly coronavirus and allow them to go back to work and school.
Now, a handful are starting or nearing the final stage of testing. Depending on the results, some companies say their vaccines could be greenlighted for use as soon as this year.
The Frontrunners
Among the first vaccine candidates to start the final round of testing is one developed by the University of Oxford and AstraZeneca. Also far along are experimental shots from Pfizer and its partner BioNTech, as well as Moderna.
China National Pharmaceutical Group Co, or Sinopharm, has a vaccine in Phase 3. A vaccine from another Chinese company, CanSino Biologics, is expected to begin the pivotal testing soon. But remember, many vaccines that show promise in early testing fail during the final round.

The Oxford/AstraZeneca vaccine is designed to provide protection by delivering into a person’s cells the genetic code for the spikes protruding from the new coronavirus. Then the cells can produce the spike proteins, generating an immune response that would be able to fight off the coronavirus. Delivering those genetic instructions is a weakened, harmless version of a virus that causes the common cold in chimpanzees.
In early testing, the vaccine successfully produced immune responses in humans with only minor side effects. A Phase 3 trial enrolling 30,000 subjects in the US began in August. Other late-stage trials are under way with several thousand volunteers in the UK, Brazil and South Africa.
•Production capacity estimate: AstraZeneca aims to make two billion doses available worldwide, and has said that one billion may be available this year.
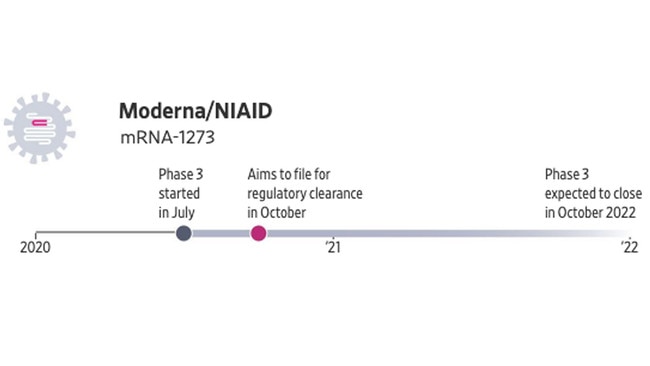
The Moderna vaccine also uses a gene-based technology to provoke an immune response, though the code it delivers takes the form of messenger RNA. Those molecules, commonly referred to as mRNA, are the body’s molecular couriers ferrying DNA instructions for making proteins. The vaccine delivers to cells mRNA for making the coronavirus’s spike protein.
Moderna and the US National Institute of Allergy and Infectious Diseases are testing a two-dose shot. It was the first candidate to enter human testing in the US. The vaccine produced an immune response in early-stage testing and was generally well-tolerated, with minor side effects observed in test subjects.
Final-stage testing is under way in the US with a 30,000-person trial that could yield interim results in the fall. An mRNA vaccine has never been approved for any disease.
• Production capacity estimate: 500 million to one billion doses a year starting in 2021.
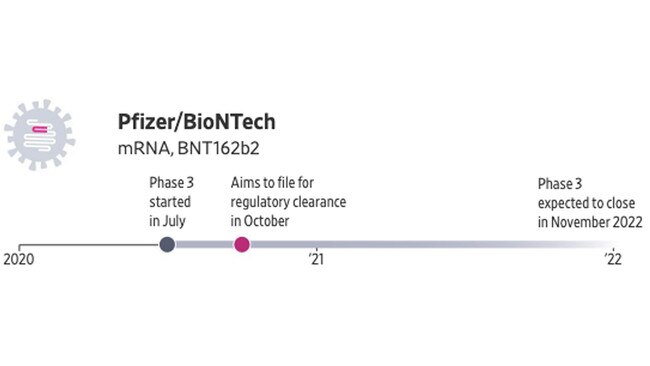
The vaccine developed by Pfizer and German partner BioNTech also uses mRNA. In a Phase 1 trial, the vaccine generated neutralising antibodies that promise to fight off the coronavirus and was generally well tolerated. Phase 3 testing began in the US in July, enrolling about 30,000 people, and will expand overseas to include about 120 sites.
The US government has agreed to pay Pfizer and BioNTech nearly $2 billion for 100 million doses. Pfizer aims to seek regulatory approval or an emergency-use authorisation in October.
• Production capacity estimate: up to 100 million doses worldwide by the end of 2020, and about 1.3 billion by the end of 2021.
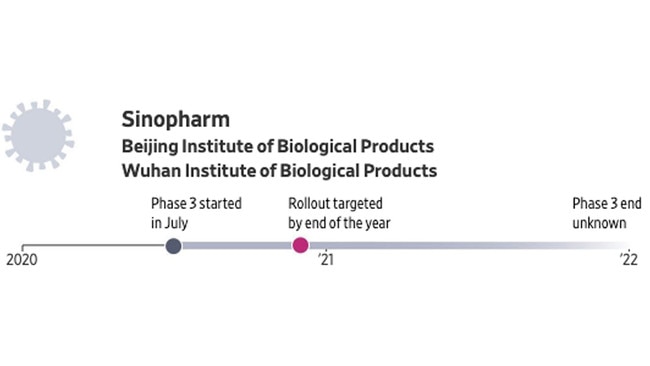
China’s state-owned Sinopharm is developing two vaccines with the government agencies Wuhan Institute of Biological Products and Beijing Institute of Biological Products. Both are based on an older vaccine-making technique.
The group has entered agreements to conduct testing in several countries, including Pakistan and the United Arab Emirates. The Wuhan Institute has drawn concern over its safety record, including over some of its vaccines for children.
The government says it started what it calls “emergency use” of some of its COVID-19 vaccines on medical workers and border inspection officials in late July. Chinese officials have said they aim to make a vaccine available to the public before the end of the year.
• Production capacity estimate: about 220 million doses a year.
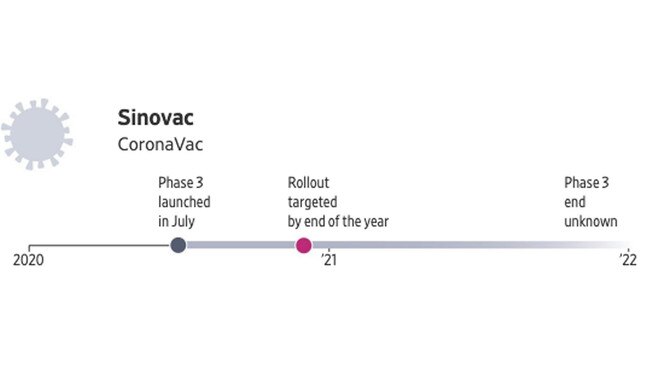
Sinovac, a private Chinese company, began its final-stage trial in July in Sao Paulo, Brazil, where it is testing its vaccine to take advantage of a higher infection rate. Sinovac has also struck a deal with Indonesian state-owned pharmaceutical holding company PT Bio Farma to make up to 250 million vaccine doses each year for the Indonesian public, according to China’s state news agency.
• Production capacity estimate: about 300 million doses a year at a Beijing plant.
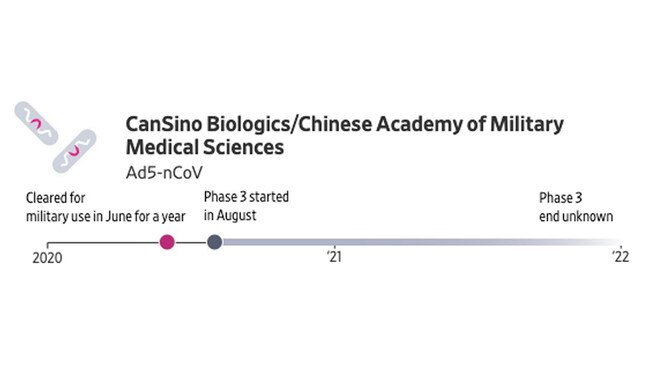
CanSino’s vaccine is aimed initially at the Chinese military. Chinese company CanSino developed the shot with the military based on a weakened virus behind the common cold. A Phase 1 study was conducted in March in Wuhan, the early epicentre of COVID-19. The shot got government clearance in June for military use for one year.
• Production capacity estimate: 100 million to 200 million doses a year starting in 2021.
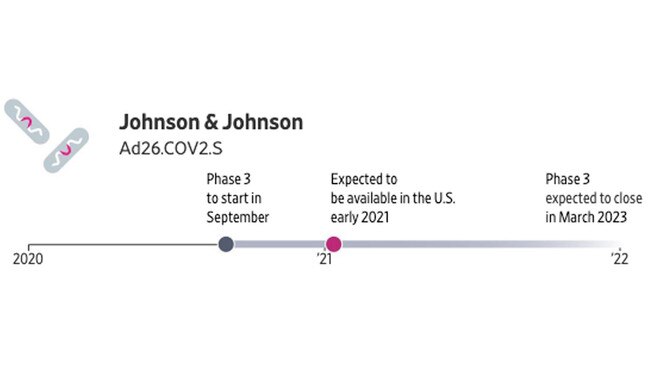
Johnson & Johnson is developing a vaccine that uses a weakened form of a common-cold virus, known as an adenovirus. A single dose of this vaccine provoked a strong immune response in early animal testing. The company plans to launch by late September a 60,000-person global study, which could be the largest late-stage clinical trial of a COVID-19 vaccine. The company will carry out the study at nearly 180 locations in the US and eight other countries where transmission rates are high, including Brazil, Chile and South Africa.
• Production capacity estimate: one billion worldwide by the end of 2021, including 100 million doses for the US, with an option for an additional 200 million, and 30 million doses for the UK, with an option for an additional purchase of up to 22 million.
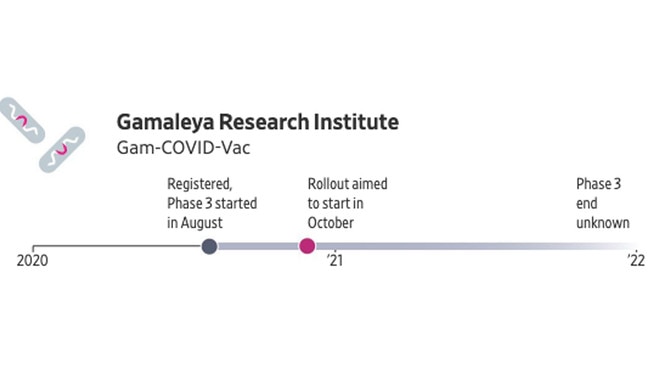
The Russian state-owned Gamaleya Research Institute is developing a vaccine based on a combination of two adenoviruses, which it has already tested on volunteers. Russia effectively approved use of the vaccine in early August, though the shot hadn’t gone through final-stage testing. The Russian government plans for mass vaccination to start in October, and will aim rollout at high-risk groups including health workers.
• Production capacity estimate: 500 million doses a year, with mass production starting September 2020.
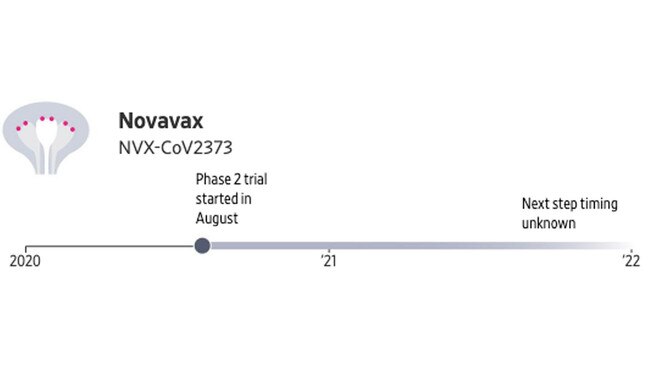
US-based Novavax is making a vaccine that consists of two shots given 21 days apart that deliver proteins resembling the spike jutting out from the new coronavirus. Researchers hope the proteins will trigger the production of antibodies and immune cells that can fight off the coronavirus.
The shots also contain a component, called an adjuvant, to boost the immune response. In Phase 1 testing, the vaccine was generally well-tolerated and produced promising numbers of antibodies. Phase 2 testing began in August, and the company has said Phase 3 could start in September.
• Production capacity estimate: 100 million doses for use in the US, with delivery beginning by the end of this year. Plans to manufacture for other countries.
The Wall Street Journal




To join the conversation, please log in. Don't have an account? Register
Join the conversation, you are commenting as Logout