Mesoblast seeks federal backing for Australian coronavirus trials
Listed biotech Mesoblast is in talks with the government about launching local trials of its experimental COVID-19 treatment.

Listed biotech Mesoblast is in talks with the federal government about launching local clinical trials of its experimental treatment to help the worst-affected COVID-19 patients fight the novel coronavirus.
The Anthony Pratt and Thorney-backed company is completing a $US30m ($46m) trial in the US and has already reported a significant increase in survival rates.
It is now planning to replicate that trial, albeit on a smaller basis, in Melbourne and Sydney. Chief executive Silviu Itescu said hospitals had already been recruited.
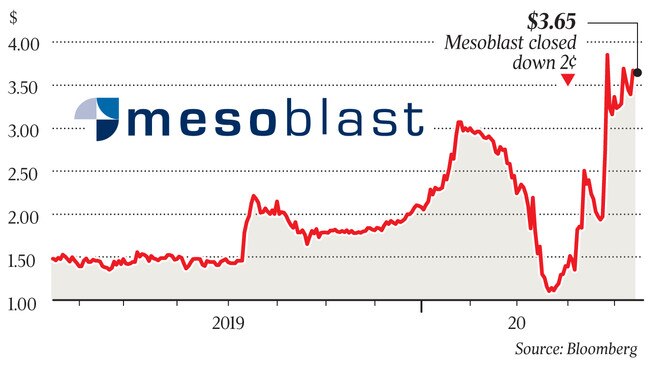
The stem-cell focused company, whose shares have soared more than 230 per cent since March 19, is now awaiting an answer from the Morrison government about funding the trial. The US government, through the National Institutes of Health, is funding the US study.
“We are already working with a number of hospital sites in Melbourne and Sydney to establish a clinical program here as well,” Dr Itescu said.
“We would like to manufacture locally in Australia for the Australian population. I think it would provide the kind of insurance we need in the event that there is a second wave, a resurgence of the virus, when the restrictions are loosened up.”
News of Mesoblast’s local trial comes as the French government rounded on homegrown pharmaceutical giant Sanofi, after its chief executive said a potential COVID-19 vaccine would go to the US first.
Sanofi chief executive Paul Hudson announced this week that people in the US would have priority because their government was helping to fund the company’s quest for a coronavirus vaccine.
“The US government has the right to the largest pre-order because it’s invested in taking the risk,” Mr Hudson said.
Dr Itescu said the US also strongly encouraged local manufacturing to serve its local population, and he hoped to gain similar support from the Morrison government.
“The US encourages local manufacturing for the US population, and we are planning for that. And it should be the same in Australia,” he said.
“We should be manufacturing locally and have products made in the event that the coronavirus gets any worse, particularly on a seasonal basis.”
While the world is sweating on a COVID-19 vaccine, which is hoped to be made available in the next year, Dr Itescu said the development of a vaccine would not completely eliminate the coronavirus, in the same way it hasn’t eradicated influenza.
He said in the US, about 60,000 people died from influenza-related respiratory distress syndrome, and Mesoblast’s treatment aimed to assist those patients as well as those with COVID-19 complications.
The treatment, called Remestemcel-L, was developed to counteract inflammation by increasing the production of naturally occurring anti-inflammatory cells to help people fight acute respiratory distress syndrome (ARDS), which had a death rate as high as 80 per cent.
Already in its US trial, Mesoblast has recorded an 83 per cent survival rate in ventilator-dependent COVID-19 patients, while 75 per cent of patients had successfully come off ventilator support within 10 days. This compares with a 12 per cent survival rate in ventilator-dependent COVID-19 patients, and 9 per cent being able to come off ventilator support within the recommended treatment time.
“Even with effective vaccines there will be a need for our therapy for viral respiratory distress syndrome, whether it’s influenza or COVID-19,” Dr Itescu said.
“We would like to manufacture here locally for the Australian population. It would be terrific if the Australian government could work with us.”
This week, Mesoblast raised $US90m to scale up manufacturing of Remestemcel-L, via a placement of 43 million shares to existing and new institutional investors at a price of $3.20.
Long-time Mesoblast investor, Melbourne billionaire Alex Waislitz, participated in the capital raising and hoped the federal government would support the company.
“Meso is now finally well on the way to becoming a major Australian success story and I’m hopeful that the Australian government will recognise Meso’s potential and encourage them to develop their stem cell manufacturing capabilities in Australia, rather than lose them to another country,” Mr Waislitz said.




To join the conversation, please log in. Don't have an account? Register
Join the conversation, you are commenting as Logout