CSL plans to build $1.8bn vaccine factory in Melbourne’s north
CSL’s influenza vaccine business, Seqirus, struck a $1.8bn deal with the Morrison government to build the factory.

CSL will look to replicate the factory it built with the US government in North Carolina when it constructs the biggest biotech and vaccine manufacturing facility in the southern hemisphere in Melbourne’s north.
The company’s influenza vaccine business, Seqirus, struck a $1.8bn deal with the Morrison government to build the factory, which is designed to ensure a rapid response to future pandemics.
While the factory will be designed to combat influenza pandemics — the North Carolina factory is already involved in a project with the US government to fight a potential bird flu outbreak — Seqirus says the Australian facility could be retooled to fight any infectious disease.
“Although the facility is being custom-built for influenza, there are parts of it that could be used if there was another pandemic with a different pathogen,” Seqirus vice-president for commercial operations Lorna Meldrum said.
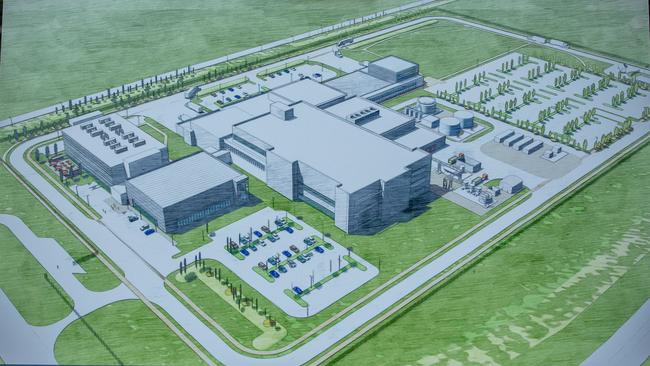
CSL will invest $800m in the factory’s construction, with the commonwealth committing to buy $1bn worth of its product, including flu vaccines, antivenoms for Australian snakes, spiders and marine creatures and a Q-Fever vaccine, over 12 years.
CSL began negotiating with the federal government three years ago to build the facility, which will be located in the Melbourne Airport Business Park.
It will take another six years for the company to build what will become the biggest facility of its kind in the southern hemisphere, by which time it is hoped the world will have recovered from COVID-19.
The depth of planning involved in the factory shows what is required to ensure adequate protection against highly infectious diseases that can shut down entire economies.
“It’s a huge construction effort, employing over 500 people. It will probably be built by the end of 2024, the start of 2025, but with any of these facilities, in making sure it is signed off by the TGA (Australian Therapeutic Goods Administration) and FDA (US Food and Drug Administration), it is about a 12-month process,” Dr Meldrum said.
“It will really be state-of-the-art, so it’s a complex build.”
This year, Seqirus completed a $US140m ($192.1m) expansion of its factory in Holly Springs, North Carolina, which it built in collaboration with the US government.
One of the North Carolina factory’s first projects has been preparing for another pandemic, which has involved the manufacture and stockpiling of AUDENZ, the first adjuvanted, cell-based influenza vaccine designed to protect against A(H5N1), commonly known as bird flu.
Dr Meldrum said the production of cell-based influenza vaccines was “a completely new way” to produce a flu vaccine, which for the past 17 years had involved using chicken eggs.
“It was a completely new way of making the vaccine and we have perfected it really, and we have continued to perfect it and improve our yields.
“In a way, the facility in the US, we will be using everything we have learned about the technique and how we have improved it. It will be a facility almost identical to the Holly Springs facility but it will be half the size.
“But we will be using all the technology, everything we have learned from that facility, to bring it here to Australia.”
Dr Meldrum said the Melbourne factory would have three components — the cell production facility for seasonal and pandemic influenza, a “high-speed” fill and finish production line which involves filling syringes with a particular vaccine, and a space to manufacture MF59 — a substance added to some vaccines to improve immune response and to reduce the amount of antigen needed for each vaccine.
“We use MF59 in our influenza vaccines but it’s also being used in the University of Queensland COVID-19 candidate.
“It does what we call antigen spare, so you need less of the antigen and therefore you can make more doses.”
CSL chief executive and managing director Paul Perreault said that providing safe and effective influenza vaccines was “essential in securing our defences against serious public health threats”.
“As a proudly Australian company, we are pleased to make this investment in world-class advanced manufacturing,” Mr Perreault said.
“This decision will ensure the future of 1000-plus science technology engineering and manufacturing jobs in Victoria and a supply chain of more than $300m annually,” he said.








To join the conversation, please log in. Don't have an account? Register
Join the conversation, you are commenting as Logout