remdesivir to be combined with anti-inflammatory drug in next phase of US trials
A drug shown to speed up the recovery of COVID-19 patients will be combined with another pill in the next phase of US trials, as the UK labelled the treatment the ‘biggest step forward’.
Coronavirus
Don't miss out on the headlines from Coronavirus. Followed categories will be added to My News.
American researchers are set to commence the next phase of clinical trials on the only drug shown to have some effect on COVID-19.
Phase two of the federal trial will combine remdesivir with another drug called baricitinib - a pill used to treat inflammation associated with rheumatoid arthritis.
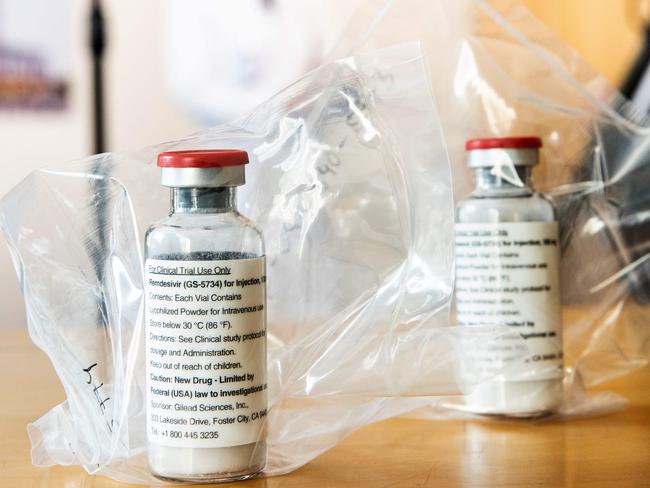
remdesivir was found to reduce the number of days COVID-19 patients spent in hospital from about 15 days on average, to 11 days, according to preliminary results from a trial published in The New England Journal of Medicine. The study of more than 1,000 patients hospitalised with the virus received either remdesivir or a placebo.
While nothing suggested the drug has a significant impact on deaths, researchers said shortened hospital stays were an important finding, according to NBC News.
“Four days being sick in a hospital receiving major care, being intubated, receiving oxygen, is an eternity,” Dr. Andre Kalil, a principal investigator for the trial at the University of Nebraska Medical Center in Omaha, told NBC News.
“The longer you stay in the hospital, the higher the chances of forming blood clots,” Dr Kalil said. “The longer you stay in a hospital, the higher the chance of getting hospital-acquired infections.”
About 600 to 700 patients are expected to take part in the next phase of the trial. All patients will be treated with remdesivir, but only half will receive baricitinib, while the other half will be given a placebo.
“If we can minimise the inflammation, and minimise the viral replication at the same time, we could further increase the clinical benefits of the patient,” Dr Kalil said.
Researchers are also weary of baricitinib’s side effects. The drug can lower the immune system, which could worsen infections. It is also known to increase platelet count.
“We need to monitor for that too, because if it gets too high, the patients could have an increased risk for developing a blood clot,” Dr Aneesh Mehta, an associate professor of medicine at the Emory University School of Medicine, told NBC News.
Dr Mehta is also a lead investigator in the trial, which is sponsored by the National Institute of Allergy and Infectious Diseases.
Mr Kalil said researchers “will not just stop”, and will “keep trying different treatments that are going to have the potential to improve patients”.
It’s expected the next set of preliminary results will be available somewhere between June and September this year.
‘BIGGEST STEP FORWARD’ HAILED IN COVID-19 TREATMENT
An experimental drug originally developed to combat ebola has been officially approved for use to treat coronavirus in what is being hailed as “the biggest step forward” in treating the virus.
The UK’s National Health Service (NHS) gave approval to use remdesivir after clinical trials showed it could help patients recover almost a third faster.
“I can announce that we’re beginning a new trial for selected NHS patients, of an antiviral drug called remdesivir,” UK Health Secretary Matt Hancock said.
“They’ve already been some promising early results on coronavirus patients, with early data suggesting you can shorten recovery time by around four days.
“As you can understand, we’ll be prioritising the use of this treatment where it will provide the greatest benefit. This is probably the biggest step forward in the treatment of coronavirus since the crisis began.”
Early data suggests it can reduce the risk of death from the virus by more than 30 per cent, but these findings are still being verified.
The drugs watchdog gave the green light for selected patients to get “early access” to the treatment.

A statement from the Medicines and Healthcare products Regulatory Agency says: “Early results from clinical studies have shown that remdesivir reduced the time of recovery from 15 days to 11 days in patients with severe COVID-19 and possibly also reduced the proportion of people dying from COVID-19.
“Therefore, remdesivir is being made available for the treatment of hospitalised patients with severe COVID-19 disease.”
Antiviral drug remdesivir, which is given via an IV drip, works by interfering with the infection’s ability to reproduce.
Its US maker Gilead will provide the treatment to the NHS free of charge.
The drug was given the green light in the US on May 1 and Japan on May 8.
Due to limited supplies, doctors will only be able to prescribe the drug to those most likely to benefit.
Innovation Minister Lord Bethell said: “This shows fantastic progress. As we navigate this unprecedented period, we must be on the front foot of the latest medical advancements, while always ensuring patient safety remains a top priority.”
Earlier this month, a global clinical trial, which is continuing, found that remdesivir cut the length of time people suffered symptoms from 15 days to 11.
The trial involves around 1,000 patients from hospitals including the UK, US, France, Italy and China.
So far in the UK, over 36,000 people have died from the virus and a major trial could help curb the outbreak.
Meanwhile, as Brazil and India struggle with surging coronavirus cases, a top health expert is warning that the world is still smack in the middle of the pandemic, dampening hopes for a speedy global economic rebound and renewed international travel.
“Right now, we’re not in the second wave. We’re right in the middle of the first wave globally,” said Dr. Mike Ryan, the World Health Organisation’s executive director.
“We’re still very much in a phase where the disease is actually on the way up,” Ryan told reporters, pointing to South America, South Asia and other areas where infections are still on the rise.
India saw a record single-day jump in new cases for the seventh straight day. It reported 6,535 new infections Tuesday, raising its total to 145,380, including 4,167 deaths.
The virus has taken hold in some of India’s poorest, most densely populated areas, underscoring the challenges that authorities face in curbing the spread of a virus for which a vaccine or cure isn’t yet in sight.
Originally published as remdesivir to be combined with anti-inflammatory drug in next phase of US trials


