World Health Organisation says AstraZeneca COVID-19 vaccine OK to use despite blood clot fears
With Europe divided over the AstraZeneca vaccine, the WHO says there is still “no link” between the vaccine and recent incidents of blood clots.

The World Health Organisation said countries should not stop using AstraZeneca’s COVID-19 vaccine amid fears about people who received the jab developing blood clots.
In a Friday briefing, WHO spokesperson Margaret Harris said no causal link had been established between the British drugmaker’s “excellent” shot and the blood clot cases that have emerged in Europe.
“It’s very important to understand that, yes, we should continue to be using the AstraZeneca vaccine,” Ms Harris said.
RELATED: Australia’s AstraZeneca vaccine rollout faces delays
The WHO’s expert vaccine advisory committee is nevertheless reviewing the reported blood clots, which have led nearly a dozen countries to suspend or delay their distribution of AstraZeneca’s vaccine.
Denmark, Norway, Iceland have stopped using the shot since Danish officials reported “severe cases” of blood clots in vaccinated people, one of which was related to a death.
Six countries including Italy and Austria halted the use of two separate batches of the vaccine over similar concerns, while Thailand has pushed back its vaccine rollout. Bulgaria also halted AstraZeneca vaccinations Friday after the death of a woman who showed no signs of blood clots.
Harris said more than 268 million doses of COVID-19 vaccines have been administered worldwide and none of them have caused any deaths. She reportedly noted that coronavirus vaccinations do not “reduce deaths from any other causes.”
“We must always ensure that we look for any safety signals when we roll out vaccines, and we must review them,” Harris said, according to the Agence France-Presse news agency. “But there is no indication to not use it.”
RELATED: Mystery remains over shipment of AstraZeneca doses in Australia
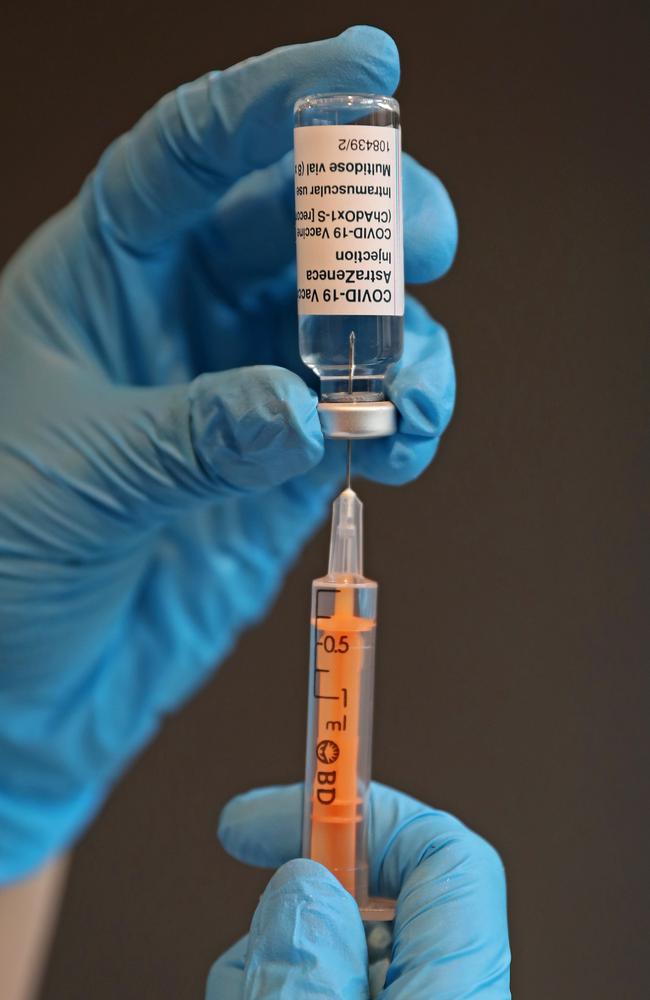
AstraZeneca has said it found no evidence of an increased risk of pulmonary embolism or deep vein thrombosis — conditions marked by the formation of blood clots — in more than 10 million records of safety data for its vaccine.
The European Union’s European Medicines Agency similarly said on Thursday that there’s no indication AstraZeneca’s shot caused the blood clots, adding that the vaccine’s benefits outweigh its risks.
AstraZeneca’s shot is expected to play a crucial role in the WHO’s COVAX initiative, which aims to distribute two billion vaccine doses this year and make sure poor countries can access vaccinations.
The Cambridge, UK-based company plans to supply 142 countries with “hundreds of millions of doses” through the initiative in the coming months, it said last week.
AstraZeneca’s US-listed shares dropped about 1.4 per cent to $US48.01 in early trading Friday.
This article originally appeared on NY Post and was reproduced with permission